Clinical Image
What next for Minimally Invasive Glaucoma Surgery (MIGS)?
Ejaz Ansari*
Department of Ophthalmology, Institute of Medical Sciences, Canterbury Christchurch University, Kent, UK
*Corresponding author: Ejaz Ansari, Department of Ophthalmology, Institute of Medical Sciences, Canterbury Christchurch University, Maidstone & Tunbridge Wells NHS Trust, Kent, UK
Published: 03 Aug, 2017
Cite this article as: Ansari E. What next for Minimally
Invasive Glaucoma Surgery (MIGS)?.
Clin Surg. 2017; 2: 1731.
Clinical Image
In their quest for safer surgery and better outcomes for their patients, glaucoma surgeons in
many parts of the world have increasingly embraced Minimally Invasive Glaucoma Surgery (MIGS)
procedures. Although generally safer than established surgical techniques, they remain a relatively
new approach and the long-term benefits for patients and cost-effectiveness are unknown.
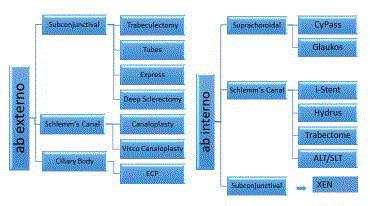
MIGS describes a broad range of procedures which could be ab interno or ab externo, either
with or without the use of implants (Figure 1). Most of the recent focus in the literature has been on
MIGS implants, which can achieve the aim of Intraocular Pressure (IOP) reduction by one of three
means: accessing the Schlemm’s Canal (SC) and collector channels; through the suprachoroidal
space and the familiar subconjunctival space. In practical terms, IOP remains the only modifiable
risk factor in glaucoma treatment. Although some implants, particularly the subconjunctival ones,
have been employed in tackling cases of advanced glaucoma, MIGS procedures generally, are
positioned between medication and trabeculectomy in the treatment paradigm. Usually, they offer
a modest reduction in IOP and medications post-operatively, and are predicated on being tissue
sparing, safer and quicker both for the surgeon and the patient, with speedier visual rehabilitation.
There are many MIGS publications, but few Randomised Controlled Trials (RCTs), a lack of
standardisation in study design and few standalone (i.e. without concomitant cataract surgery)
studies. However, some interesting observations on efficacy and safety can be drawn from the
current literature, with emphasis on the RCTs and comparative studies that are available.
Trabecular meshwork bypass stents, include iStent (Glaukos Corp., Laguna Hills, CA, USA),
which has been in use for several years and is FDA approved. Since most MIGS procedures are
combined with cataract extraction and lens implant (CE+IOL), it is difficult to elucidate the effect of
the implant alone. Therefore, studies comparing combined procedures with CE+IOL are important.
One RCT showed a significant reduction in IOP and medications compared to cataract surgery
alone [1]. There is evidence that potentially accessing multiple collector channels with more than
one iStent is more efficacious in IOP reduction [2]. This has spawned the development of a second
generation iStent, the iStent Inject/ model GST400 (Glaukos Corp., Laguna Hills, CA, USA), which
is pre-loaded with 2 stents. Favourable reductions in IOP and medications were demonstrated in
standalone studies, with follow-up of 12 and 18 months [3,4] and in a 4 year combined study [5].
These studies were non-randomised and non- comparative.
Hydrus (Ivantis Inc, Irvine, CA, USA)- is another trabecular meshwork bypass stent that
straddles 3 clock hr of SC, with the aim of accessing more collector channels and dilating the SC,
acting as a scaffold so that it does not block the collector channel ostia.
A RCT of 100 cases randomised to cataract surgery alone or combined cataract surgery with
Hydrus, has been completed [6]. At 24 months, a significantly greater proportion of combined
surgery cases reached the endpoint of 20% reduction in washed out diurnal IOP and the IOP was
also significantly lower in the combined surgery group. There was also a significant reduction in
cases without ocular hypotensive medications in the combined surgery group.
Implants accessing the suprachoroidal space include the CyPass (Alcon, Fort Worth, TX,
USA), which is FDA approved and is delivered ab interno, forming a conduit between the anterior
chamber and suprachoroidal space. A 24 months RCT of 505 subjects randomized to CE/IOL+
CyPass or CE/IOL alone demonstrated fairly similar IOP lowering of 7.4 mmHg compared to 5.4
mmHg in the control group, but the difference being statistically significant (p< 0.001), with 85% of
treated patients drop-free at 24 months [7]. In a 12 month standalone study, CyPass achieved a 35% reduction in IOP at 12 months with a reasonable reduction in drop dependency [8].
Subconjunctival implants appear to have superior efficacy
compared to other devices. However, there is a lack of high quality
evidence for this group of implants. The XEN gel stent (Allergan,
Dublin, Ireland) is an ab interno gelatine stent that is implanted
via a clear corneal incision without conjunctival dissection. Three
models have been evaluated with inner diameters of 45 μm, 63 μm,
and 140 μm, with the 45μm being the recommended device by the
manufacturer. XEN 45 is FDA approved.
Initial studies with the 63 and 140 model, demonstrated
favourable IOP lowering with reduced medication burden, but these
studies were not randomised nor were they comparative, usually
with a mixture of combined and standalone cases [9,10]. The IOP
reduction with XEN 45 was 25% in a small 12 month study, with
mainly combined cases [11]. The results from the ongoing APEX study
(combined procedures, open angle glaucoma, and 2 year follow-up)
demonstrated a mean reduction in IOP of 39% (mean IOP at 2 years
of 13.1 mmHg) with a mean of 0.7 medications. However, the rate of
needling significantly exceeded that seen following trabeculectomy.
The results will be published later this year.
The Innfocus microshunt (Santen Pharm Ltd, Osaka, Japan)
is an ab externo drainage device targeting the subconjunctival
space. It involves more steps akin to trabeculectomy compared to
other MIGS. Long-term IOP reduction is encouraging with 80%
reaching IOP < /=14 mmHg at 3 years, some cases being standalone
and others combined. The mean IOP for the entire group was 10.7
+/-1.5 mmHg at 3 years; qualified success rate was 95% with a
reduction in medications from 2.6+/-0.9 to 0.8+/-1.2 [12]. Therefore,
subconjunctival procedures appear to be more efficacious (Table 1),
but inevitably require more post-operative bleb management, and
potentially restrict future incisional procedures if required.
MIGS procedures have come a long way but are still evolving.
More work is required to evaluate these procedures, particularly
with more randomised controlled trials, standalone and comparative
studies and cost-effectiveness modelling, before they can find a place
in the glaucoma surgery firmament. With time, as the technology
evolves further, clinicians will be better informed about the place of
MIGS in their practice. Standardisation and improvements in the
quality of future MIGS studies will help clinicians to negotiate this
ever-expanding area more knowledgably and help them to optimise
the selection of the appropriate device for the right patient. With the
correct approach to investigating and evaluating new technologies,
there is much potential for future generations of MIGS procedures to
improve the quality of care for glaucoma patients.
Figure 1
Table 1
References
- Craven ER, Katz LJ, Wells JM, Giamporcaro JE; iStent Study Group. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild to moderate open angle glaucoma and cataract: Two year follow-up. J Cat Ref Surg. 2012;38(8):1339-45.
- Katz LJ, Erb C, Carceller GA, Fea AM, Voskanyan L, Wells JM, et al. Prospective randomised study of one, two, or three trabecular bypass stents in open angle glaucoma subjects on topical hypotensive medication. Clin Ophthalmol Auckl NZ. 2015;9:2313-20.
- Voskanyan L, Garcia-Feijoo J, Belda JI, Fea A, Anselm J, Christophe B. Prospective masked evaluation of the iStent inject system for open-angle glaucoma: synergy trial. Adv Ther. 2014;31(2):189-201.
- Lindstrom R, Lewis R, Hornbeak Dana M, Lilit V, Jane EG, John H, et al. Outcomes following implantation of two second generation Trabecular Micro-Bypass Stents in Patients with POAG. Adv Therapeutics. 2016;33(11):2082-90.
- Arriola-Villalobos P, Martinez-de-la-Casa JM, Diaz-Valle DJ, Laura MF, Cristina FP, Julian GF. Glaukos iStent inject® Trabecular Micro-Bypass Implantation Associated with Cataract Surgery in Patients with Coexisting Cataract and Open-Angle Glaucoma or Ocular Hypertension: A Long-Term Study. J Ophthalmol. 2016;2016:1056573.
- Pfeiffer N, Garcia-Feijoo J, Martinez-de-la-Casa JM, Larrosa JM, Fea A, Lemij H, et al. A randomized trial of a Schlemm’s canal microstent with phacoemulsification for reducing intraocular pressure in open-angle glaucoma. Ophthalmology. 2015;122(7):1283-93.
- Vold S, Ahmed IIK, Craven ER, Mattox C, Stamper R, Packer M, et al. Two-Year COMPASS Results: Supraciliary Microstenting with Phacoemulsification in Patients with Open-Angle Glaucoma and Cataracts. Ophthalmology. 2016;123(10):2103-11.
- García-Feijoo J, Rau M, Grisanti S, Hoh H, Erb C, Ahmed I, et al. Supraciliary micro-stent implantation for open-angle glaucoma failing topical therapy: 1-year results of a multicenter study. Am J Ophthalmol. 2015;159(6):1075-81.
- Sheybani A, Lenzhofer M, Hohensinn M, Reitsamer H, Ahmed II2. Phacoemulsification combined with a new ab interno gel stent to treat open-angle glaucoma: Pilot study. J Cataract Refract Surg. 2015;41(9):1905-9.
- Sheybani A, Dick B, Ahmed IIK. Early clinical results of a novel ab interno gel stent for the surgical treatment of open-angle glaucoma. J Glaucoma. 2016;25(7):e691-6.
- Galal A, Bilgic A, Eltanamly R, Osman A. XEN glaucoma implant with mitomycin C 1 year follow-up: result and complications. J Ophthalmol. 2017; 2017:5457246.
- Batlle JF, Fantes F, Riss I, Pinchuk L, Kato YP, Parel JM, et al. Three year follow-up of a novel aqueous humor microshunt. J Glaucoma. 2016;25(2):e58.