Review Article
Evolution of a System to Increase Precision in the Surgical Management of Colorectal Carcinoma
Charles L Hitchcock1*, Thomas J Magliery2, Cathy Mojzisik3, Morgan Johnson4, Mark W Arnold5
and Edward W Martin,5
1Department of Pathology, Ohio State University, 1645 Neil Ave Columbus, OH 43210, USA
2Department of Chemistry & Biochemistry, Ohio State University, Columbus, OH 43210 100W 18th Ave, USA
3Clinical Development, Enlyton Ltd. 1216 Kinnear Rd Columbus, OH 43212, USA
4Department of Medicine, Ohio State University College of Medicine, 370 W 9th Ave Columbus, OH 43210, USA
5Department of Surgery, Ohio State University, 410 W 10th Ave Columbus, OH 43210, USA
*Corresponding author: Charles L Hitchcock, Department of Pathology, Ohio State University, 1645 Neil Ave Columbus, OH 43210, USA
Published: 06 Jun, 2017
Cite this article as: Hitchcock CL, Magliery TJ, Mojzisik C,
Johnson M, Arnold MW, Martin EW.
Evolution of a System to Increase
Precision in the Surgical Management
of Colorectal Carcinoma. Clin Surg.
2017; 2: 1491
Abstract
Current surgical procedures for colorectal adenocarcinoma are plagued by a lack of precise
information provided by preoperative imaging and the surgeon’s exploration of the surgical field
using traditional techniques (i.e., inspection and palpation). The staging of colorectal adenocarcinoma
begins with preoperative imaging and ends with the pathologist; however, potential sources of error
between these two points may result in suboptimal treatment impacting outcome. Using colorectal
adenocarcinoma as a model, we developed a System incorporating currently available technologies
to increase the precision of tumor imaging before and during surgery as well as intraoperative
tumor detection.
The multimodal System focused on the patient evolved over 35 years. The System brings together
essential resources (i.e., molecular probes, imaging modalities and detection devices) and expertise
of various clinical specialties (i.e., Nuclear Medicine, Oncology, Pathology, Radiology, Radiation
Oncology and Surgery) for precision diagnosis and optimal treatment.
Although the diagnosis and treatment of colorectal adenocarcinoma was the focus throughout the
System’s development, it is applicable to other adenocarcinomas.
Developing the System to increase the precision in the surgical management of colorectal
adenocarcinoma began with the selection of the tumor-related antigen, tumor associated
glycoprotein-72 (TAG-72). Generations of anti-TAG-72 monoclonal antibodies radiolabeled with
125I were safely used as molecular probes. During surgery, a hand-held gamma probe was used for
the detection and excision of TAG-72 positive tissues. Long term follow-up of patients with primary
colorectal adenocarcinoma demonstrated a survival advantage in those who TAG-72 “Status
@ Closing” was negative. Our proof-of-concept studies demonstrated that this System increases
the surgical precision, and thus the quality of care, for individual patients. The proposed use of
bioengineered anti-TAG-72 monoclonal antibody fragments radiolabeled with 123I for hand-held
gamma probe detection along with pre- and post-resection intraoperative gamma imaging would
more precisely answer the question, “Did you get it all?” for those patients with colorectal and other
adenocarcinomas.
Introduction
The need for precision
Over 1,685,000 new cancers will be diagnosed in the U.S. in 2016, excluding keratinocyte
carcinoma. Of these, approximately 85% will be carcinomas with adenocarcinomas making up
the majority. Adenocarcinomas of the colon and rectum constitute 134,490 of these. However, the
prevalence of adenocarcinomas is four times the incidence rate, which equates to 621,430 patients
living with colorectal carcinoma in 2016 [1].
The National Comprehensive Cancer Network (NCCN) developed guidelines and clinical
resources to help physicians treat, detect, prevent, reduce risk, provide supportive care, and image
a large number of different cancers, including colorectal adenocarcinoma [2]. The TMN staging
criteria forms the platform for the guideline for colorectal carcinomas, and its accuracy is critical to treatment selection and planning. The staging of these tumors begins
with preoperative imaging and ends with the pathologist, but there
are many potential sources of error between these two points that
can impact patient treatment and outcome. In the case of colorectal
carcinomas, despite these evidence based guidelines, more than
40% of patients who underwent a “curative resection” of a primary
tumor will have recurrent disease, and patients with the same stage
of colorectal adenocarcinoma can differ in their clinical course. The
reason is a lack of precision.
The National Institutes of Health (NIH) defines the term
“Precision Medicine” as “an emerging approach for disease treatment
and prevention that takes into account individual variability in
genes, environment, and lifestyle for each person [3].” In the case
of colorectal adenocarcinoma, the complete removal of all tumorbearing
tissue requires precision in the localization and detection of
intraabdominal metastatic disease before and during surgery. There
are several factors that impact this precision.
The NCCN guidelines call for using computerized tomography
(CT) scans with contrast for preoperative imaging, needed for surgical
planning for resection of primary and recurrent disease. This includes
respectability of the primary tumor and assessment of the presence
of metastatic disease that alters the surgical approach or mandates
non-surgical therapies. Despite providing anatomic information,
poor spatial resolution has an adverse effect on the specificity and
sensitivity of conventional and contrast CT imaging to detect lymph
nodes smaller than 5 mm that often contain metastatic disease [4].
The end result is a wide range of reported specificity from only 42%
to 70% [5-9].
Patients often ask “Did you get it all?” Current surgical procedures
are based on surgical anatomy and traditional planes of resection that
are easily violated by cancer cells. Variation in surgeon experience
influences the type of tumor resection and surgical precision.
Traditional visual and manual evaluation does not necessarily provide
surgeons with intra-operative information needed to obtain, not just
for a curative resection, but a resection that actually cures. As one
of us has previously noted, “[I]f surgeons had real-time information
regarding the precise location of all disease and had a real-time
assessment of surgical resection margins, they would be able to
intervene immediately to accomplish a complete resection without
subjecting the patient to subsequent additional surgical procedures
[10].”
Advances in Precision Medicine are getting underway. This paper
examines how our diverse group of physicians, basic scientists and
engineers brought currently available resources and developed new
ones that have evolved over 35 years into a multimodal System that
provides the surgeon with the approach and tools needed to increase
the precision of tumor imaging and detection, before and during
surgery, for the individual patient with a solid tumor. Although our
focus is on colorectal adenocarcinoma, the proposed system applies
to the majority of adenocarcinomas that arise in other organs.
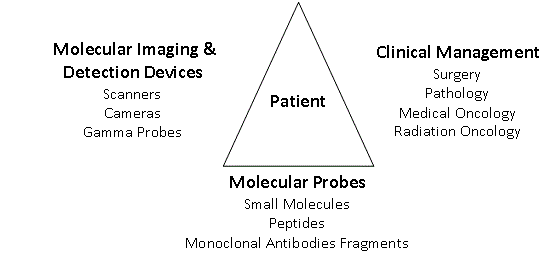
Figure 1
A System to Increase Precision Management of Colorectal Cancer Patients
System components
The components of our multimodal System are seen in Figure
1. With the patient at its center, the System integrates physicians
from Nuclear Medicine, Radiology, Surgery, Oncology, Radiation
Oncology and Pathology with the tools needed for a more precise
diagnosis and treatment of the patient’s cancer. Molecular probes,
specific for the patient’s tumor, are the foundation of the System.
Based on the results of the initial biopsy and/or laboratory studies,
the Pathologist recommends the appropriate molecular probe to
be used. Labeling of the molecular probe is dictated by the type of
molecular imaging and intraoperative detection devices. The results
of molecular imaging determine optimal treatment, such as surgery or
undergoing chemotherapy and/or radiation therapy. Precise imaging
provides the surgeon with a “mine field map” of where to go with the
intraoperative hand-held gamma-detection probe to find and remove
the tumor containing tissue. Intraoperative imaging provides realtime
verification of complete resection. From a systems standpoint,
the complete resection of all tumors is globally cost effective.
The System begins with the patient and their solid tumor. The
tumor’s pathologic features are used to select the appropriate tumor
specific or associated molecular probe and radionuclide or nonradioactive
label. The labeled-molecular probe dictates the type of
devices that can be used for preoperative and intraoperative imaging
and for intraoperative detection. The results of imaging and/or
intraoperative detection will aid in treatment decision making before
and/or after tissue examination by Pathology.
Molecular probes
In contrast to the anatomic information provided by CT and
MRI imaging, molecular imaging is a diagnostic modality that
provides functional information about molecular makeup of tissue.
Molecular imaging uses a variety of radiolabeled molecular probes
for positron emission tomography (PET) and single-photon emission
computed tomography (SPECT) alone or in combination with CT or
magnetic resonance imaging (MRI). Constantly evolving, molecular
imaging provides the necessary versatility needed for the System’s
multimodality approach to increasing the precision of cancer surgery
[11]. There are several categories of tumor-related molecular probes
available for molecular imaging [12]. They include small molecules
that bind intracellular targets, small peptides that bind to membrane
receptors, and monoclonal antibodies (MAbs) and bioengineered
MAb fragments that bind to tumor-related antigens. Ongoing studies
are directed at the production of molecular probes that rapidly enter
the tumor and bind to the specific target in the tumor, lack uptake
by non-target tissue, and rapidly clear from the blood and normal
tissue. The end result of this optimization is a reduction in unwanted
background that will yield the maximum signal-to-noise for the
probe [13].
Categorized as a small molecule molecular probe, [18F]-2-fluoro-
2-deoxyglucose (18F-FDG) is widely used for preoperative PET or
PET/CT imaging of patients with cancer, monitoring patients for
recurrent disease, and more recently for assessing response to therapy
[14]. However, 18F-FDG is not cancer specific. As a glucose analog, FDG is taken up into cells with a high metabolic rate. This includes cells
within tumors, normal organs (e.g., brown fat, myocardium, brain,
gastrointestinal (GI) tract, thyroid, liver and spleen), inflammatory
responses (e.g., infections granulomas and immune hyperplasia), and
wound healing. In addition, FDG accumulates in the kidneys and
bladder prior to its excretion in the urine [15]. These result in false
positive findings. In addition, tumors with a low metabolic rate do
not take up FDG. False negative PET and PET/CT scans often occur
with invasive bronchioloalveolar carcinoma and carcinoid tumors in
the lung, renal cell carcinomas, hepatomas, mucinous tumors of the
gastrointestinal tract, and low grade non-Hodgkin lymphomas. The
false positive and false negative rates call into question the precision
of 18F-FDG PET and 18F-FDG PET/CT for pre- or perioperative
staging of tumors [16,17]. The reported sensitivity of 18F-FDG PET/
CT for the detection of lymph node metastases is reported as low as
43% for colorectal carcinomas, which is below the needed diagnostic
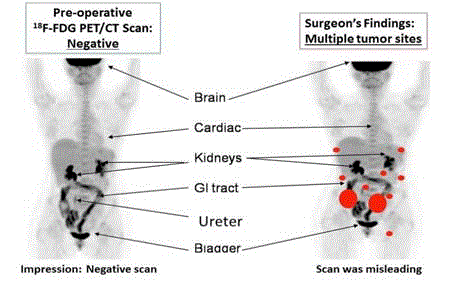
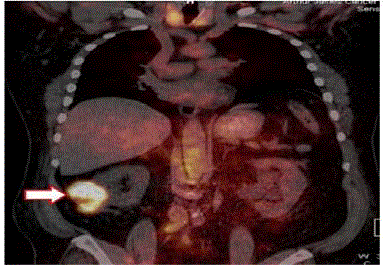
precision for our System (Figure 2) [18].
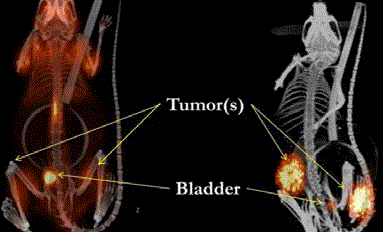
The left image is the pre-operative PET/CT scan that was
interpreted as negative for cancer. Nonspecific uptake of the 18F-FDG
was present in the brain and GI tract and compared to the kidneys,
ureter and bladder where the molecular probe accumulated. The right
image correlates the surgical findings (orange dots) with the same
18F-FDG-PET/CT scan. This false negative finding led to unneeded
surgery.
Small peptides of no more than 15 amino acid are used for both
SPECT and PET molecular imaging, alone or in combination with
CT. These molecular probes act as ligands for various membrane
receptors, the most common of which is the multiple types of
somatostatin receptors on neuroendocrine tumors (NETs). They
have excellent specificity, stability, and low immunogenicity, but
are prone to proteolysis [12,19,20]. Radionuclide labeling generally
requires an intermediate chelator attached to the peptide. As most
gastrinomas and other foregut neuroendocrine tumors (NETS) over
express somatostatin receptors, somatostatin receptor imaging using
SPECT/CT is the method-of-choice for pre- and/or perioperative
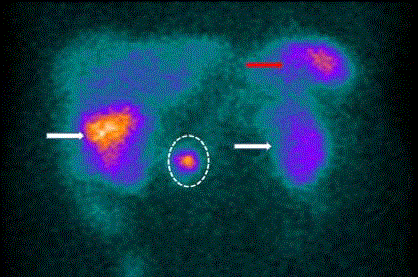
staging of gastrinomas (Figure 3) [21,22]. However, the advent of
PET/CT probes will replace them in the future, especially for midgut
and hind gut NETs [23].
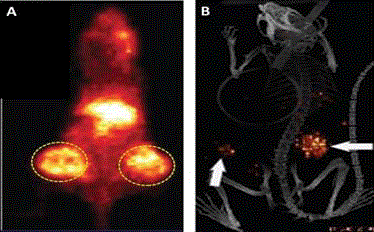
Large field-of-view gamma camera (SPECT) scan of 111Inpentetreotide
bound to somatostatin receptors on a gastrinoma
cells (dotted circle). There is non-specific uptake in the spleen (red
arrow) and accumulation in the gallbladder (right white arrow), and
in the kidneys (left kidney- white arrow, right kidney behind the
gallbladder). Note the poor spatial resolution.
Monoclonal antibodies (MAb), directed against tumor-related
antigens, are being developed as molecular probes for molecular
imaging and/or therapy. The efficacy of a given MAb is limited by
the type of tumor(s) and the level of expression of the target antigen.
Ideally, MAb molecular probes exhibit the properties listed on
Table 1. Several different MAbs have been approved by the FDA for
molecular imaging [12], the majority of which have been labeled for
SPECT imaging. However, numerous other monoclonal antibodies
and their bioengineered counterparts are working their way through
the clinical trial steps needed for Food and Drug Administration
(FDA) approval for molecular imaging both SPECT 123I and PET 124I
modalities.
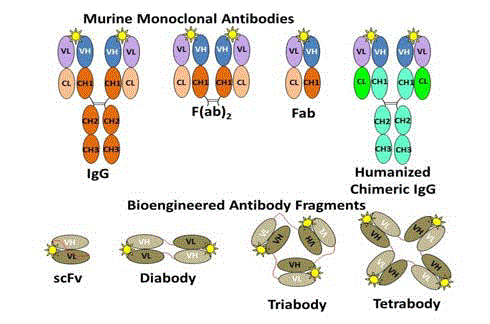
The development of MAbs to meet these desired properties
resulted in multiple generations of monoclonal antibodies and their
biochemical and genetic engineered protein fragments of antibody
molecules (Figure 4). The clinical utility of first generation intact
murine IgG molecules was limited by their large size, accumulation in non-target tissue, long serum half-lives, and immunogenicity that
induced the formation of human anti-mouse antibodies (HAMA).
Their slow clearance and slow uptake in the target tissue necessitated
that they be labeled with radionuclides with longer half-lives [111In (2.8
days), 89Zr (3.3 days), 124I (4.2 days), or 125I (60 days)] [24]. Enzymatic
digestion of the intact IgG molecules gave rise to smaller F(ab)2 and
Fab fragments with better pharmacokinetics; however, these were
still immunogenic. Genetic engineering directed at minimizing
the immunogenicity resulted in chimeric IgG molecules (Figure 4)
that contain amino acids of the murine variable regions attached to
the human constant regions. Fully humanized MAbs (not shown)
containing only 5% murine-derived amino acids from the antigen
binding site [25].
Development of MAb fragments for the desired properties for a
given application resulted in small single-chain variable fragments
(scFv) and their diabodies. The scFv is monomeric with a 12–15
amino acid linker (Figure 4 - red line) between the VH and the VL
domains. Linker composition and length can have a significant
impact on antigen binding and stability. Diabodies contain two
non-covalently associated scFv-like fragments that interact with and
bind to their corresponding antigen in a divalent manner. Tribody
and tetrabody molecules of these scFv fragments are also possible.
The scFv fragments of bispecific diabodies (not shown) have different
antigen binding specificities. When compared to intact IgG, F(ab)2
and Fab fragments, scF vs. and diabodies have faster clearance with
excellent tumor penetration, and higher tumor-to-blood ratios. The
result is low background and a high signal-to-noise ratio resulting in
increased precision of molecular imaging to identify malignant tissue
[26,27].
Tuning antibody fragments to the exact molecular imaging
application remains a significant frontier for engineering and
development. The fragment size can be adjusted by genetic
engineering, linker manipulation, and chemical modification (for
example with PEG, an inert polymer of ethylene glycol), but often
these modifications result in poor stability, poor or ablated binding,
and aggregation. But adjustments in fragments size translate into
adjustments in clearance time suitable for different imaging time
lines, modalities and sensitivities.
Yellow star represents the same antigenic epitope attached to
the antigen binding site of each of the MAb molecules and their
fragments. The murine variable regions are fused to human constant
regions to give rise to the humanized and chimeric IgG molecules.
The scFv and its corresponding diabody, triabody, and tetrabody
variants can contain only a few murine-derived amino acids, which
that are essential for antigen binding. The peptide linker between the
domains provides many of the desired physical properties of these
molecular probes for imaging.
Figure 2
Figure 3
Figure 4
Figure 5
Table 1
Molecular Imaging & Intraoperative Detection Devices
Detection of tumor-related molecular probes depends on the
use of a wide range of radionuclides and non-radioactive labels. The
half-life of the radionuclide must be matched to the half-life of the
molecular probe to optimize imaging and timing of surgery. As an
example, if a particular molecular probe is slow to clear from the
blood and normal tissue, then the imaging is delayed for several days
or weeks, and the radioisotope with a shorter half-life would not
be detected. PET imaging require positron emitting radionuclides,
whereas SPECT imaging directly detects photons from gamma
emitters.
High energy (511 KeV) radionuclides such as 18F, 124I or 68Ga emit
positrons that annihilate electrons, giving rise to two photons that
travel in opposite directions and are detected by the PET scanner.
PET instruments contain multiple gamma cameras arranged in a
circular fashion. More often than not, PET is combined with CT for
anatomical information. Lower energy radionuclides such as 123I,
99mTc, and 111In emit γ-radiation which is detected using planar or
tomographical γ-cameras (SPECT). The ability to perform whole
body scans and obtain multiple images over time is a major advantage
of these types of molecular imaging. The limitless depth of penetration
associated with the imaging use of radionuclide-labeled molecular
probes induces a loss of spatial resolution due to the inverse square
law of intensity as a function of distance. Combining CT or MRI
with PET or SPECT along with the ongoing development of new
generations of tumor–specific MAbs will only increase the precision
of molecular imaging by providing both anatomic and more precise
functional localization of primary and metastatic malignancies. As an
example, tumor–specific MAbs labeled with high energy molecular
probes have been shown to provide high specificity and sensitivity in
detecting tumors in patients with clear cell renal cell carcinoma [28]
(Figure 5).
PET/CT 124I MAb cG250 (arrow) with clear cell renal cell
carcinoma in the lower pole of the right kidney. Focal molecular
probe also labels the thyroid glands.
Hand-held gamma probes (HGPs), and to a lesser extent
laparoscopic gamma detecting probes, are used for intraoperative
detection of radiation that is unbound or bound to a molecular probe [24,29,30]. Widely available, these probes are either like a gamma
camera, or they are solid state detectors containing a semiconductor
crystal. Our studies have primarily employed a HGP containing
a cadmium telluride (CdTe) crystal linked to a control unit that
provides both numerical information and an auditory signal when the
radioactivity is above three standard deviation above the background
radiation [31]. Their precision for routine use in radioguided surgery
is operator dependent. The surgeon may not go outside of the planned
surgical field or they may not be aware of the instruments restricted
field of view or that the sensitivity and specificity increases as the
probe moves closer to the source of radiation [24]. The precision of
the surgeon, using the HGDP, is enhanced by utilizing intraoperative
portable gamma camera that provides real-time intraoperative
localization of the low-energy radionuclide labeled molecular probes.
The use of these nuclear medicine instruments allows the surgeon in
real-time to determine the success of the operation and whether or
not he or she “got it all.”
Commercially available fixed gamma cameras collect the low
energy emission to produce a planar image that can be used in
surgery to provide real-time images. Small gamma cameras are handheld
and are easily used for intraoperative imaging. However, these
instruments take 10-60 seconds to generate an image which may be
less than optimal due to an unsteady hand. Larger, portable, gamma
cameras require stabilization and can have either a small field of view
(5 × 5 cm2) or large field of view ( >5 × 5 cm2) such as seen in Figure
3 [32]. We and others have used intraoperative gamma cameras
for intraoperative imaging of sentinel lymph nodes, parathyroid
adenomas, and a variety of tumors including: gastrinomas, head-andneck
squamous cell carcinomas, breast cancer, and melanoma [28,32-
34]. Gamma cameras have a larger field of view than the HGDP, and
thus provide the surgeon with a unique visual assessment of the
extent of disease and its complete resection.
Table 2
Table 3
A System Engineered to Increase Surgical Precision for Colorectal Carcinoma
Based on initial conventional imaging studies, up to 80% of
patients with colorectal adenocarcinoma lack clinical stage IV disease
and undergo curative surgery with or without adjuvant therapy. (Table
2) However, more than 40% of these patients will have recurrent
disease, which primarily occurs in the lymph nodes, liver and/or
lungs. The best survival potential for patients undergoing curative
surgery for colorectal adenocarcinoma is the complete removal of all
tissue containing tumor. One has to remember the adage that it’s not
what the surgeon removes during surgery that kills the patient, but it is
what is left behind (residual cancer).
The proposed “System” brings together the surgeon, radiologist,
nuclear medicine physician, and pathologist in order to increase
the precision of “getting it all.” Using molecular imaging, they
identify where the malignant tumor sites are and intraoperatively
refine the “map” to ensure that the surgeon does a more complete
resection. Increasing the precision of intraoperative detection of
tumor will increase the pathologist’s ability to “physiologically,” as
well as anatomically, stage the tumor. In the last 35 years, our group
generated several lines of evidence supporting this clinical claim,
especially for colorectal adenocarcinomas.
The “System” begins with the selection of the most appropriate
tumor-related antigen. For colorectal carcinoma we selected Tumor
Associated Glycoprotein-72 (TAG-72). TAG-72 is an oncofetal
antigen that is expressed by the majority of human adenocarcinomas
(Table 3). TAG-72 is a large mucin-like molecule consisting of 80%
carbohydrate moieties [27].
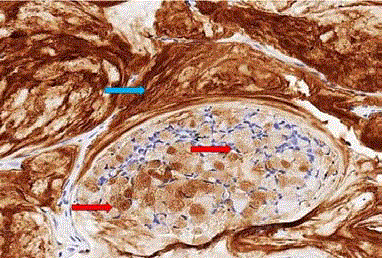
Immunohistochemical staining for TAG-72 (Figure 6)
demonstrates these molecules in cytoplasmic vacuoles of the tumor cells that release the molecule into the lumen of tumor acini and
into the extracellular matrix where it accumulates. The extracellular
accumulation of TAG-72 facilitates its targeting by radiolabeledantibodies
and subsequent localization by molecular imaging. These
features results in the ideal target molecule for molecular imaging and
intraoperative detection.
The TAG-72 molecule is a complex array of different antigenic
epitopes, to which multiple MAbs have been developed [38]. Of
these, we selected B72.3 murine MAb and its subsequent generations.
The evolution of antibodies to TAG-72 followed the prescribed path
previously noted for MAbs as molecular probes (Table 4). The initial
four generations of antibodies to TAG-72 were generated in the
same laboratory at the NIH [39-41], and were used by us to increase
the precision of radioimmunoguided surgery (RIGS) in an attempt
to detect all tumor in real-time and to remove it from patients with
either primary or recurrent colorectal adenocarcinoma [29].
Clinical studies using the first three generations of the murine
anti-TAG-72 MAbs were complicated by several factors. The
immunogenicity of murine IgG molecules resulted development
HAMA, whose only clinical significance was interference with several
clinical laboratory tests [42]. That fact that these were whole IgG
molecules with a long half-life required labelling with 125I with halflife
of 60 days. These resulted there being a delay of up to four weeks
before surgery was performed and a tumor-background (signalnoise)
ratio of 2:1. The somewhat smaller size of the 3rd generation
MAb to TAG-72 doubled the tumor-background ratio and halved its
clearance time to allow for an improved time to surgery, and did not
induce significant HAMA [43,44].
Numerous clinical studies have used one of these first three
generations of 125I-labelled MAbs to TAG-72 to study over 1,000
patients with either primary or recurrent adenocarcinomas, with
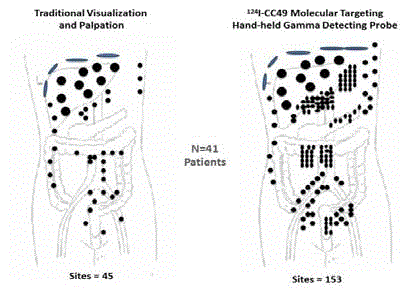
a focus on colorectal carcinomas. (Reviewed in 29, 31) Figure 7
demonstrates the increased precision by which the surgeon can
detect remove TAG-72 containing metastatic disease using a HGDP
as compared to that obtained by traditional visual inspection and
palpation [45]. These findings, found in numerous other studies
[46-50], had significant impact in altering clinical decision making
in up to 50% of cases. These decisions included abandoning surgery
due to extensive disease (e.g., carcinomatosis), increasing the area of
resection, and up-staging leading to adjuvant chemotherapy [45-54].
The increased precision that intraoperative detection and
removal of occult metastatic disease provides a significant survival
advantage to patients with primary colorectal adenocarcinoma.
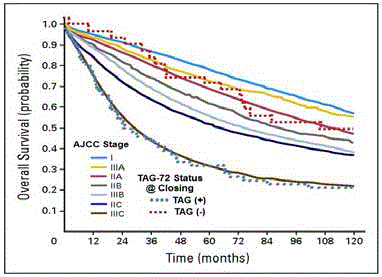
(Figure 8) A longitudinal follow-up of 97 patients with primary
colorectal adenocarcinoma demonstrated that patient survival at 5,
10, and 15 years [31,55,56] was significantly improved when all of the
TAG-72 positive tissue was surgically removed. The TAG-72 “Status
@ Closing” is a bimodal, real-time, intraoperative assessment of the
patient’s survival potential at the time of closing that is independent
of the TNM stage. The survival of those patients in the TAG (+)
category mimics that of patients with Stage IIIC disease. In contrast,
those patients where all TAG-72 containing tissue was removed,
classified as TAG (-), regardless of the TNM stage, the survival was
consistent with disease confined to the bowel wall with or without
minimal nodal involvement.
Based on current AJCC TNM staging criteria, the solid lines
represent the 10-year survival for 128,853 primary colon carcinoma
patients in the SEER Database [57]. Using the presence [TAG (+ - blue
dotted line)] or absence [(TAG (-) – red dotted line] of radioactivity
at the time of closing (TAG-72 Status @ Closing) the dotted lines
represent survival data from 97 patients that were given 125I-CC49 and
subsequently underwent HGDP directed intraoperative detection with possible resection of radioactive tissue [56].
TAG-72 positive tissues that lack evidence of tumor on routine
H&E staining is considered to be a false-positive finding [50,58].
However, several lines of evidence indicate that this is a misconception.
Clinically, the data in Figure 8 indicates that all TAG-72 positive
tissue, regardless of H&E staining status, has clinical significance if
left behind. Secondly, the non-regional periportal lymph nodes often
contain TAG-72 activity with the HGDP. Subsequent recurrent
disease was found in these nodes if they had been previously respected
[59]. Just as important, routine pathologic examination of these
“false positive” lymph nodes lacks precision. Additional sections
submitted for H&E staining and/or immunohistochemical staining
demonstrated metastatic disease; however, the detection sensitivity
of the light microscope appears to have its limits as well [60-62].
More sensitive molecular studies detected metastatic cells where the
microscope could not [63,64].
Many of these previous studies were complicated by the use of
the first three generations of murine MAbs to TAG-72. They were
potentially immunogenic and their large molecular size resulted in
poor pharmacokinetics and the need for 125I labelling with its less
than optimal long half-life that delayed surgery up to four weeks after
injection [27,63]. Despite these obvious disadvantages, the precision
in the surgical management of colorectal adenocarcinoma, as well
as other tumors, can be further increased by using the previously
mentioned (above) multimodal approach, where the tumor-related
antigen TAG-72 is targeted using 5th generation scFv or other
fragment MAbs, labelled with radionuclides 123I or 124I with their short
half-lives. The excellent pharmacokinetics of these molecules provide
little background to impair preoperative and/or perioperative,
molecular imaging while facilitating next-day-surgery using a HGDP
and intraoperative and post-operative molecular imaging.
This can be accomplished by targeting TAG-72 using humanized
single chain Fv fragments (scFv) and its bi- tri- and tetravalent forms
(Figure 4). These smaller molecules retain the specificity and affinity
of the previous generation murine CC49 (unpublished data). Their
small size optimizes their pharmacokinetics, yielding molecular
imaging with a much higher signal-to-noise (i.e., tumor-tobackground)
ratio (unpublished data) as well as providing for same
day surgery and intraoperative detection. Studies with xenografts
human adenocarcinoma cells clearly demonstrate 18F-FDG and the
humanized 4th generation MAb to TAG-72 (3E8), lack the precision
obtained using humanized 3E8 fragment, a 5th generation MAb to
TAG-72 (Figure 9 and 10).
The clinical significance of this proposed approach has been
addressed in recent proof-of-concept (POC) studies that combined
pre- and perioperative molecular imaging with intraoperative
imaging and the use of a HGDP to ensure that the surgeon “got it
all.” Gastrinomas often characterized by their over expression of
somatostatin receptors on their membrane which bind the peptide
ligand 111In-labeled octreotide as a molecular probe for imaging. A
proof-of-concept study clearly demonstrated that this probe can be
used for preoperative SPECT/CT followed by planar imaging with
a portable large field-of-view gamma camera (LFOVGC) before
incision, and at the completion of surgery, intraoperatively. The
precision of the surgery was furthered by the intraoperative use of a
HGDP for locating primary and metastatic tumor [28,34]. A second
POC study used the same approach for the molecular imaging 99mTc-
Sestamibi (MIMI) binding to parathyroid adenomas in 20 patients
[33]. Although a benign disease, primary hyperparathyroidism
requires the resection of the related parathyroid adenomas to
prevent development of debilitating sequelae. Resection of involved
gland is often complicated by its anomalous location in the neck
and mediastinum. The portable LFOVGC was again used to ensure
complete resection prior to closure. The resulting increase in precision
significantly decreased time in the operating room by reducing the
need to confirm complete resection by delaying Parathyroid hormone
(PTH) studies until the patient was in recovery [65].
Table 4
Figure 6
Figure 7
Figure 7
Intraoperative Tumor Location of Metastatic Disease: Traditional
Visualization and Palpation vs. Hand-Held Gamma Detection Probe
(HGDP) of Occult Tumor Binding 125I-CC49 in 41 cases of primary colorectal
adenocarcinoma [45].
Figure 8
Figure 9
Figure 10
Figure 10
124I-IgG 3E8 PET scan vs. 124I-diabody 3E8 PET/CT Scan of
Human Colon Cancer Xenografts in Mice.
Conclusion
The current guidelines for colorectal cancer surgery do not
take into account the limited precision of visual inspection and
palpation, and even CT scans, to accurately detect nodal metastases
outside of the traditional planes of dissection, which clearly have a
significant influence on patient survival. Although used for molecular
imaging and for HGDP intraoperative localization and resection of
malignant tissue, the use of 18F-FDG-PET/CT for molecular imaging
lacks the necessary precision needed to identify these same lymph
nodes. The identification and excision of these malignant lymph
nodes requires a multimodal System. As proposed here, this System
brings together the necessary resources and the expertise of various
clinical specialties needed to present the surgeon with real-time,
intraoperative, information needed to locate, identify and resects all
malignant tissue expressing the radiolabeled molecular probe. That
is, the System provides a map of the “tumor’s mine field” and the
position of the “malignant mines” within it that will allow for their
safe removal. Two small proof-of-concept studies used this approach
with great success; however, these studies require expansion. The
model system for these expanded studies should be one where the
number of potential patients is large and the clinical impact can
be determined with statistical confidence. We propose that such a study be undertaken with primary colorectal adenocarcinomas that
examine the role of the proposed System on making colorectal cancer
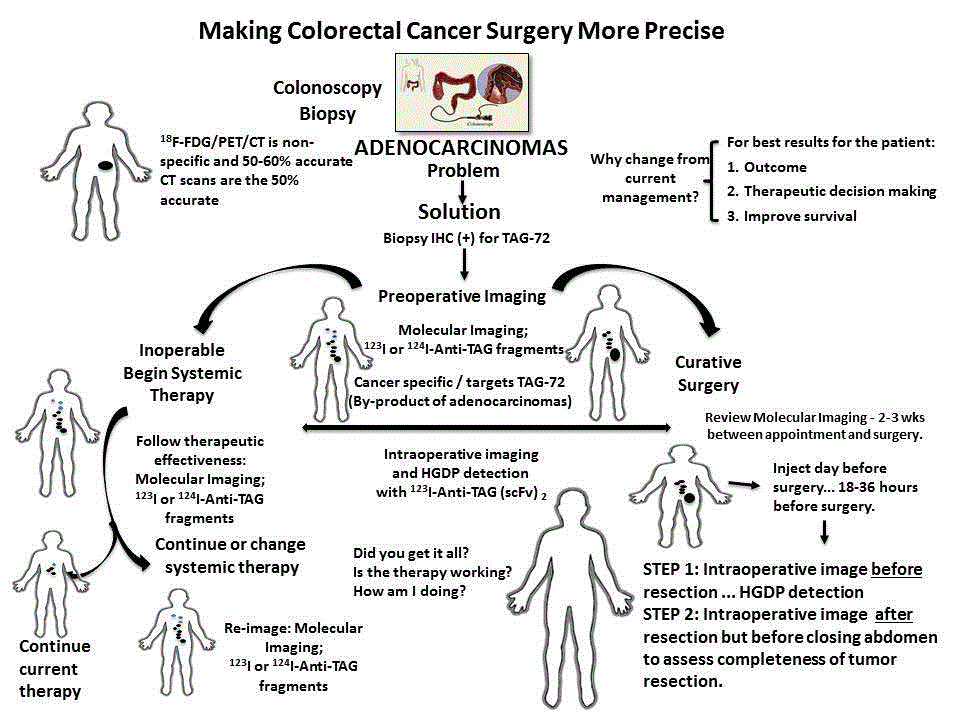
surgery more precise (Figure 11).
Patient presenting with sign and symptoms of colorectal
cancer undergo laboratory studies, including CEA serum levels,
and colonoscopy with a biopsy of all relevant lesions. If an invasive
adenocarcinoma is noted, the pathologist with perform IHC staining
to determine the presence or absence of TAG-72 expression. The
fact that TAG-72 is expressed in 85% of colorectal adenocarcinomas
makes anti-TAG-72 the ideal foundational molecular probe for the
System in these patients. If the initial biopsy is shown to express TAG-
72, the patient is injected with a 124I- or 123I-anti-TAG-72 antibody
fragment and imaged using PET/CT or PET/MRI, or SPECT/CT,
respectively. The results of this molecular imaging determine if the
patient can undergo surgery for cure or undergo chemotherapy and/
or radiation therapy.
If clinically resectable, the day before surgery the patient is given
a 123I-anti-TAG fragment cocktail to facilitate localization of TAG-72
antigen-expressing malignant tissue. Intraoperative use of a HGDP in
conjunction with a portable LFOVGC allows the surgeon to precisely
identify all TAG-72 positive tissue, including surgical margins for
excision, and to ensure that it is excised. Prior to closing, a planar
image will tell the surgeon the patient’s TAG-72 status at closing.
This real-time intraoperative information about each tissue specimen
will be available to the pathologist to aid in clearly identifying where
to sample the respected specimens for subsequent processing and
microscopic examination. In addition, this information will be
available for more precise post-operative treatment planning before
the patient leaves the recovery room. Where molecular imaging
demonstrated inoperable disease, the patient is referred to an
oncologist for treatment planning that may include chemotherapy
and/or radiation therapy. Here again the molecular imaging using
either 124I or 123I labeled anti-TAG-72 fragments will be used to follow
therapeutic effectiveness. In the end, increased precision leads to
increased quality of patient care.
Figure 11
References
- Rasooly RS, Gossett DR, Henderson MK, Hubel A, Thibodeau SN. High-Throughput Processing to Preserve Viable Cells: A Precision Medicine Initiative Cohort Program Workshop. Biopreserv Biobank. 2017.
- Herrera-Ornelas L, Justiniano J, Castillo N, Petrelli NJ, Stulc JP, Mittelman A. Metastases in small lymph nodes from colon cancer. Arch Surg. 1987;122(11):1253-6.
- Thoeni RF. Colorectal cancer. Radiologic staging. Radiol Clin North Am. 1997;35(2):457-85.
- Jeune F, Brouquet A, Caramella C, Gayet M, Abdalla S, Verin AL, et al. Cardiophrenic angle lymph node is an indicator of metastatic spread but not specifically peritoneal carcinomatosis in colorectal cancer patients: Results of a prospective validation study in 91 patients. Eur J Surg Oncol. 2016;42(6):861-8.
- Jeune F, Brouquet A, Caramella C, Gayet M, Abdalla S, Verin AL, et al. Cardiophrenic angle lymph node is an indicator of metastatic spread but not specifically peritoneal carcinomatosis in colorectal cancer patients: Results of a prospective validation study in 91 patients. Eur J Surg Oncol. 2016;42(6):861-8.
- Jeune F, Brouquet A, Caramella C, Gayet M, Abdalla S, Verin AL, et al. Cardiophrenic angle lymph node is an indicator of metastatic spread but not specifically peritoneal carcinomatosis in colorectal cancer patients: Results of a prospective validation study in 91 patients. Eur J Surg Oncol. 2016;42(6):861-8.
- Dighe S, Purkayastha S, Swift I, Tekkis PP, Darzi A, A'Hern R, et al. Diagnostic precision of CT in local staging of colon cancers: a meta-analysis. Clin Radiol. 2010;65(9):708-19.
- Wiegering A, Kunz M, Hussein M, Klein I, Wiegering V, Uthe FW, et al. Diagnostic value of preoperative CT scan to stratify colon cancer for neoadjuvant therapy. Int J Colorectal Dis. 2015;30(8):1067-73.
- de Vries FE, da Costa DW, van der Mooren K, van Dorp TA, Vrouenraets BC. The value of pre-operative computed tomography scanning for the assessment of lymph node status in patients with colon cancer. Eur J Surg Oncol. 2014;40(12):1777-81.
- Edward W. Martin Jr. In: Herrmann K, Nieweg OE, Povoski SP, editors. Radioguided Surgery: Current Applications and Innovative Directions in Clinical Practice (pages vii-ix). Cham, Heidelberg, New York, Dordrecht, and London: Springer. 2016.
- Weber J, Haberkorn U, Mier W. Cancer stratification by molecular imaging. Int J Mol Sci. 2015;16(3):4918-46.
- James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev. 2012; 92:897-965.
- Frangioni JV. The problem is background, not signal. Mol Imaging. 2009;8(6):303-4.
- Brush J, Boyd K, Chappell F, Crawford F, Dozier M, Fenwick E, et al. The value of FDG positron emission tomography/computerised tomography (PET/CT) in pre-operative staging of colorectal cancer: a systematic review and economic evaluation. Health Technol Assess. 2011;15(35):1-192.
- Long NM, Smith CS. Causes and imaging features of false positives and false negatives on 18F-PET/CT in oncologic imaging. Insights Imaging. 2011;2(6):679-98.
- Lu YY, Chen JH, Ding HJ, Chien CR, Lin WY, Kao CH. A systematic review and meta-analysis of pretherapeutic lymph node staging of colorectal cancer by 18F-FDG PET or PET/CT. Nucl Med Commun. 2012;33(11):1127-33.
- Park K, Jang G, Baek S, Song H. Usefulness of combined PET/CT to assess regional lymph node involvement in gastric cancer. Tumori. 2014;100(2):201-6.
- Gade M, Kubik M, Fisker RV, Thorlacius-Ussing O, Petersen LJ. Diagnostic value of (18) F-FDG PET/CT as first choice in the detection of recurrent colorectal cancer due to rising CEA. Cancer Imaging. 2015;15(1):11.
- Andreas K, Ulrich K. Use of radioactive substances in diagnosis and treatment of neuroendocrine tumors. Scand J Gastroenterol. 2015;50(6):740-7.
- Johnbeck CB, Knigge U, Kjaer A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: current status and review of the literature. Future Oncol. 2014;10:2259-77.
- Béhé M, Gotthardt M, Behr TM. Imaging of gastrinomas by nuclear medicine methods. Wien Klin Wochenschr. 2007;119(19-20):593-6.
- Termanini B, Gibril F, Reynolds JC, Doppman JL, Chen CC, Stewart CA, et al. Value of somatostatin receptor scintigraphy: a prospective study in gastrinoma of its effect on clinical management. Gastroenterology. 1997;112(2):335-47.
- Jimenez Londoño GA, García Vicente AM, Soriano Castrejon AM, Gómez López OV, Palomar Muñoz A, Vega Caicedo CH, et al. Role of 99mTc-HYNIC-Tyr3-octreotide scintigraphy in neuroendocrine tumors based on localization of the primary tumor. Minerva Endocrinol. 2016;41(1):10-18.
- Povoski SP, Neff RL, Mojzisik CM, O'Malley DM, Hinkle GH, Hall NC, et al. A comprehensive overview of radioguided surgery using gamma detection probe technology. World J Surg Oncol. 2009:7:11.
- Kaur S, Venktaraman G, Jain M, Senapati S, Garg PK, Batra SK. Recent trends in antibody-based oncologic imaging. Cancer Lett. 2012;315(2): 97-111.
- Wu AM. Engineered antibodies for molecular imaging of cancer. Methods. 2014;65(1):139-47.
- Povoski SP, Mojzisik CM, Sullivan BJ. Radioimmunoguided Surgery: Intraoperative Radioimmunodetection for the Radioguided Localization and Resection of Tumors. In: Herrmann K, Nieweg OE, Povoski SP, editors. Radioguided Surgery: Current Applications and Innovative Directions in Clinical Practice. Cham, Heidelberg, New York, Dordrecht, and London: Springer. 2016: 371-417.
- Povoski SP, Hall NC, Murrey DA Jr, Sharp DS, Hitchcock CL, Mojzisik CM, et al. Multimodal imaging and detection strategy with 124 I-labeled chimeric monoclonal antibody cg250 for accurate localization and confirmation of extent of disease during laparoscopic and open surgical resection of clear cell renal cell carcinoma. Surg Innov. 2013;20(1):59-69.
- Povoski SP. The History of Radioguided Surgery: Early Historical Milestones and the Development of Later Innovative Clinical Applications. In: Herrmann K, Nieweg OE, Povoski SP, editors. Radioguided Surgery: Current Applications and Innovative Directions in Clinical Practice. Cham, Heidelberg, New York, Dordrecht, and London: Springer. 2016: 3-12.
- Barrio AV, Cody HS III. Radioguided Sentinel Lymph Node Mapping and Biopsy in Breast Cancer. In: Herrmann K, Nieweg OE, Povoski SP, editors. Radioguided Surgery: Current Applications and Innovative Directions in Clinical Practice. Cham, Heidelberg, New York, Dordrecht, and London: Springer. 2016: (115-123).
- Sun D, Bloomston M, Hinkle G, Al-Saif OH, Hall NC, Povoski SP, et al. Radioimmunoguided surgery (RIGS), PET/CT image-guided surgery, and fluorescence image-guided surgery: past, present, and future. J Surg Oncol. 2007;96(4):297-308.
- Hellingman D, Vidal-Sicart S. The Use of Intraoperative Small and Large Field of View Gamma Cameras for Radioguided Surgery. In: Herrmann K, Nieweg OE, Povoski SP, editors. Radioguided Surgery: Current Applications and Innovative Directions in Clinical Practice. Cham, Heidelberg, New York, Dordrecht, and London: Springer. 2016: 35-56.
- Hall NC, Plews RL, Agrawal A, Povoski SP, Wright CL, Zhang J, et al. Intraoperative Scintigraphy Using a Large Field-of-View Portable Gamma Camera for Primary Hyperparathyroidism: Initial Experience. BioMed Res Int. 2015;2015:930575.
- Hall NC, Nichols SD, Povoski SP, James IA, Wright CL, Harris R, et al. Intraoperative Use of a Portable Large Field of View Gamma Camera and Handheld Gamma Detection Probe for Radioguided Localization and Prediction of Complete Surgical Resection of Gastrinoma: Proof of Concept. J Am Coll Surg. 2015; 221(2):300-8.
- Siegel R, Ma J, Zou Z, Jemal A. Cancer Statistics, 2014. CA Cancer J Clin. 2014;64(1):9-29.
- Julien S, Videira PA, Delannoy P. Sialyl-Tn in cancer: How did we miss the target? Biolmolecules. 2012;2:435-66.
- Molinolo A, Simpson JF, Thor An, Schlom J. Enhanced tumor binding using immunohistochemical analyses by second generation anti-Tumor-associated Glycoprotein 72 monoclonal antibodies versus monoclonal antibody B72.3 in human tissue. Cancer Res, 1990;50:1291-8.
- Kuroki M, Fernsten PD, Wunderlich D, Colcher D, Simpson JF, Poole DJ, et al. Serological mapping of the TAG-72 tumor-associated antigen using 19 distinct monoclonal antibodies. Cancer Res. 1990;50(16):4872-9.
- Colcher D, Horan Hand P, Nuti M, Schlom J. A spectrum of monoclonal antibodies reactive with human mammary tumor cells. Proc Nati Acad Sci. 1981;78(5):3199-203.
- Sheer DG, Schlom J, Cooper HL. Purification and Composition of the Human Tumor-associated Glycoprotein (TAG-72) Defined by Monoclonal Antibodies CC49 and B72.3. Cancer Res. 1998;48(23):6811-18.
- Muraro R, Kuroki M, Wunderlich D, Poole DJ, Colcher D, Thor A, et al. Generation and characterization of B72.3 second generation monoclonal antibodies reactive with tumor-associated glycoprotein 72 antigen. Cancer Res. 1988;48(16):4588-96.
- Sosolik RC, Hitchcock CL, Becker WJ. Heterophilic antibodies produce spuriously elevated CK-MB concentrations in a selected patient population. Am J Clin Pathol. 1997;107(5):506-10.
- Agnese DM, Abdessalam SF, Burak WE Jr, Arnold MW, Soble D, Hinkle GH, et al. Pilot study using a humanized CC49 monoclonal antibody (HuCC49DeltaCH2) to localize recurrent colorectal carcinoma. Ann Surg Oncol. 2004;11(2):197-202.
- Fang L, Holford NH, Hinkle G, Cao X, Xiao JJ, Bloomston M, et al. Population pharmacokinetics of humanized monoclonal antibody HuCC49?CH2 and murine antibody CC49 in colorectal cancer patients. J Clin Pharmacol. 2007;47:227-37.
- Arnold MW, Hitchcock CL, Young DC, Burak WE Jr, Bertsch DJ, Martin EW Jr. Intra-abdominal patterns of disease dissemination in colorectal cancer identified using radioimmunoguided surgery. Dis Colon Rectum. 1996;39(5):509-13.
- Burak WE Jr, Schneebaum S, Kim JA, Arnold MW, Hinkle G, Berens A, et al. Pilot study evaluating the intraoperative localization of radiolabeled monoclonal antibody CC83 in patients with metastatic colorectal carcinoma. Surgery. 1995;118(1):103-8.
- Arnold MW, Schneebaum S, Berens A, Petty L, Mojzisik C, Hinkle G, et al. Intraoperative detection of colorectal cancer with radioimmunoguided surgery and CC49, a second-generation monoclonal antibody. Ann Surg. 1992;216(6):627-32.
- Arnold MW, Schneebaum S, Berens A, Mojzisik C, Hinkle G, Martin EW Jr. Radioimmunoguided surgery challenges traditional decision making in patients with primary colorectal cancer. Surgery. 1992;112(4):624-30.
- Haddad R, Avital S, Troitsa A, Chen J, Baratz M, Brazovsky E, et al. Benefits of radioimmunoguided surgery for pelvic recurrence. Eur J Surg Oncol. 2001;27:298-301.
- Schneebaum S, Troitsa A, Haddad R, Avital S, Kashtan H, Baratz M, et al. Immunoguided lymph node dissection in colorectal cancer: a new challenge? World J Surg. 2001;25(12):1495-8.
- Avital S, Haddad R, Troitsa A, Kashtan H, Brazovsky E, Gitstein G, et al. Radioimmunoguided surgery for recurrent colorectal cancer manifested by isolated CEA elevation. Cancer. 2000;89(8):1692-8.
- Nieroda CA, Mojzisik C, Sardi A, Ferrara PJ, Hinkle G, Thurston MO, Martin EW Jr. Radioimmunoguided surgery in primary colon cancer. Cancer Detect Prev. 1990;14(6):651-6.
- Percivale P, Bertoglio S, Meszaros P, Schenone F, Gipponi M, Moresco L, et al. Radioimmunoguided surgery with different iodine-125 radiolabeled monoclonal antibodies in recurrent colorectal cancer. Semin Surg Oncol. 1998;15(4):231-4.
- Sickle-Santanello BJ, O’Dwyer PJ, Mojzisk C, Tuttle SE, Hinkle GH, Rousseau M, et al. Radioimmunoguided Surgery Using the Monoclonal antibody B72.3 in colorectal tumors. Dis Colon Rectum. 1987;30(10):761-4.
- Bertsch DJ, Burak WE Jr, Young DC, Arnold MW, Martin EW Jr. Radioimmunoguided surgery for colorectal cancer. Ann Surg Oncol. 1996;3(3):310-6.
- Povoski SP, Hatzaras IS, Mojzisik CM, Arnold MW, Hinkle GH, Hitchcock CL, et al. Antigen-directed cancer surgery for primary colorectal cancer: 15-year survival analysis. Ann Surg Oncol. 2012;19(1):131-8.
- SEER Cancer Statistics Factsheets: Colon and Rectum Cancer. National Cancer Institute. Bethesda.
- Cornelius EA, West AB. False tumor-positive lymph nodes in radioimmunodiagnosis and radioimmunoguided surgery: etiologic mechanisms. J Surg Oncol. 1996;63(1):23-35.
- Schneebaum S, Arnold MW, Houchens DP, Greenson JK, Cote RJ, Hitchcock CL, et al. The significance of intraoperative periportal lymph node metastasis identification in patients with colorectal carcinoma. Cancer. 1995;75(12):2809-17.
- Greenson JK, Isenhart CE, Rice R, Mojzisik C, Houchens D, Martin EW Jr. Identification of occult micrometastases in pericolic lymph nodes of Duke's B colorectal cancer patients using monoclonal antibodies against cytokeratin and CC49. Correlation with long-term survival. Cancer. 1994;73(3):563-9.
- Cote RJ, Houchens DP, Hitchcock CL, Saad AD, Nines RG, Greenson JK, et al. Intraoperative detection of occult colon cancer micrometastases using 125 I-radiolabled monoclonal antibody CC49. Cancer. 1996;77(4):613-20.
- Hitchcock CL, Sampsel J, Young DC, Martin E Jr, Arnold MW. Limitations with light microscopy in the detection of colorectal cancer cells. Dis Colon Rectum. 1999;42(8):1046-52.
- Martinez DA, Barbera-Guillem E, LaValle GJ, Martin EW Jr. Radioimmunoguided surgery for gastrointestinal malignancies: an analysis of 14 years of clinical experience. Cancer Control. 1997;4(6):505-16.
- Hitchcock CL, Arnold MW, Young DC, Schneebaum S, Martin EW Jr. TAG-72 expression in lymph nodes and RIGS. Dis Colon Rectum. 1996;39(4):473-5.
- Povoski SP, Murrey DA Jr, Hall NC. 18F-FDG-Directed Surgery and 18F-FDG-Directed Interventional. In: Herrmann K, Nieweg OE, Povoski SP, editors. Radioguided Surgery: Current Applications and Innovative Directions in Clinical Practice. Cham, Heidelberg, New York, Dordrecht, and London: Springer. 2016: 421-445.