Review Article
Enterocutaneous Fistula: Evidence-based Management
Ryan P Dumas1*, Sarah A Moore1 and Carrie A Sims2
1Department of Traumatology, Surgical Critical Care & Emergency Surgery, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA
2Department of Surgery, Division of Traumatology, Surgical Critical Care & Emergency Surgery, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA
*Corresponding author: Ryan P Dumas, Department of Traumatology, Surgical Critical Care & Emergency Surgery, Perelman School of Medicine at the University of Pennsylvania, 51 N. 39th Street, MOB Suite 120, Philadelphia, PA 19104, USA
Published: 26 Apr, 2017
Cite this article as: Dumas RP, Moore SA, Sims CA.
Enterocutaneous Fistula: Evidencebased
Management. Clin Surg. 2017;
2: 1435.
Abstract
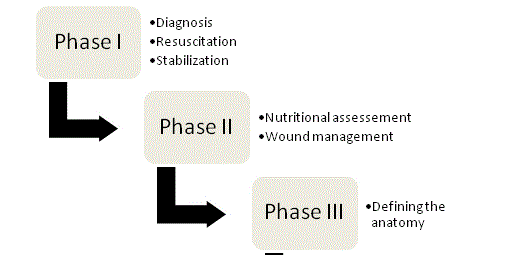
The management of Enterocutaneous fistula (ECF) is a clinical skill that should be in the armamentarium of every general surgeon. Although definitive treatment frequently relies on surgical closure, pre-operative care and diligence is paramount to ensure a successful outcome. Care of these patients should focus on four keys phases. The first phase is characterized by appropriate recognition and resuscitation. During the second phase, a complete nutritional assessment and plan is undertaken. Radiographic evaluation during the third phase helps to define ECF anatomy. Finally, the fourth phase is characterized by definitive closure should the fistula fail to heal spontaneously. This review will focus on the key aspects that define each phase and help the general surgeon maximize chances for a positive outcome.
Introduction
The development of an Enterocutaneous fistula (ECF), defined as an anomalous connection
between the bowel lumen and external skin, is a significant source of morbidity and mortality despite
advances in both surgical and medical care. The overall incidence of ECF, however, is unknown
with the majority of data regarding ECF coming in the form of large institutional retrospective or
surgeon-specific series [1-7]. Remarkably, only 25% of all ECF’s are secondary to inflammatory bowel
disease, diverticular pathology, trauma, radiation and malignancy [8]. In contrast, nearly 75% of all
ECF’s are the direct result of either laparoscopic or open surgery with an astomotic leak following
enterectomy being responsible for over 50% of these fistulas. Inadvertent iatrogenic injuries such as
enterotomies or unrecognized thermal injury sustained during the course of an operation account
for the remaining fistulas [8,9]. Because ECF is most commonly a surgeon-created problem, the
onus of prevention as well as appropriate management, planning and definitive treatment rightfully
falls onto the surgeon.
Morbidity and mortality following ECF is exceedingly high. An estimated 90% of patients will
experience an ECF-related morbidity ranging from skin excoriation, to dehydration, to sepsis.
Moreover, the mortality attributable to an ECF ranges anywhere from 5-20% and is dependent
on number of factors including underlying infection and fistula location [1,3,10-12]. Because the
leading cause of death in patients with ECF is sepsis, source control remains one of the cornerstones
of therapy. In retrospective series, mortality was noted to increase 16-fold in the setting of sepsis and
22-fold in the setting of uncontrolled infection [11,13]. As such, early and definitive treatment of un
drained collections in conjunction with short-term antimicrobials has the potential to significantly
improve outcomes. In most series, mortality also appears to correlate with fistula output and
location. Mortality increases from 26% in low output fistulas to 50% for high output ones given
the fluid, electrolyte and nutritional challenges associated with ECF management [14]. Mortality
also correlates with location and decreases with more distal fistulas. While jejunal fistulas have the
highest mortality at 29%, and are significantly more challenging to manage, the mortality from
colonic fistula is the lowest at 6% [11].
Beyond the patient impact, which ranges from both physiologic to psychologic, [15] ECF
management places a tremendous strain on healthcare resources. It is estimated that the cost of
ECF management is well over $500,000 and requires a multi-disciplinary team of nutritionists,
wound care nurses and surgeons in order to ensure a good outcome [16,17]. The importance of
high-expertise treatment centers should not be under-estimated and has been shown to significantly
decrease mortality from 42% to 20% [18].
Table 1
Table 2
Classification
There is no universal or well-established classification scheme for ECFs. Fistulas are generally classified anatomically, physiologically or by disease process [8,19]. Fistulas can additionally be classified by the quantity of daily output. It is imperative that fistula output be accurately documented as the amount may dictate changes in management. Table 1 details three different classification paradigms.
Prognostic Factors for ECF
The prognostic factors for ECF have been very well described
and are consistent with standard surgical dogma. Patients with
a proximal, high output fistula accompanied with a low albumin
(< 3.0g/dl) have more complications and are less likely to close their
fistula spontaneously [11]. Conversely patients with no comorbidities
who have fistulas that are the result of a surgical procedure and are
low output do more favorably with higher spontaneous closure
rates [13]. Table 2 details many of the variables that may predict a
successful outcome.
The importance of institutional support and multidisciplinary
expertise as a positive prognostic factor cannot be over-emphasized.
Multiple studies have confirmed institutional experience and volume
as critical factors in decreasing morbidity and improving mortality
[1,5,20]. Recently, the Canadian Association for Enterostomal
Therapy codified the importance of multidisciplinary care by
identifying nine essential team members in their “ECF Best Practice”
recommendations. Ranging from surgeons to pharmacists to
chaplains, this integrated team approach recognizes the expertise
each specialty brings to caring for these complex patients [21].
Diagnosis
A smoldering post-operative course complicated by ileus and the development of a post-operative wound infection is a harbinger for ECF. Once a fistula is suspected or has been identified on physical examination, radiologic studies are necessary adjuncts and 97% of all patients undergo some form of radiologic evaluation [1]. Contrastedenhanced CT scanning is essential as it evaluates for the presence of an associated abscess or un drained collection. Once source control has been optimized, fluoroscopic fistulography plays an important role in defining the fistula anatomy. A fistulogram will help to locate the origin, determine the length, evaluate for the presence of distal obstruction and determine whether the fistula is in continuity with the rest of the bowel [22]. Once the diagnosis of an ECF has been confirmed, focus should shift to management.
Figure 1
Management
The tenets of ECF management were first championed by Chapman and colleagues in 1964 [23] and to-date they remain the cornerstones of therapy. In their original article, Chapman et al. identified four key factors: fluid resuscitation, source control, effluent management and skin protection. The importance of nutrition has recently emerged as a fifth key element. An organized, systematic approach should be undertaken with every fistula patient in order to optimize chances of a successful outcome; and management can ultimately be broken down into the four phases (Figure 1).
Resuscitation
Resuscitation of a patient with a newly diagnosed ECF follows many of the same principle as the resuscitation of septic patients and tenets of the Surviving Sepsis Campaign should serve as a frame work [24]. Initial care should focus on aggressive fluid resuscitation, rapid assessment and correction of electrolyte imbalances, and normalization of lactic acidosis. Patients with ECF are commonly hyponatremic, hypokalemic, and acidotic due to ongoing GI losses. Patients with high output fistulas should have fluid, electrolyte and bicarbonate losses replaced intravenously in order to avoid dehydration and profound metabolic instability during the initial stabilization period. Careful monitoring of urine output and targeted replacement of fistula effluent every 4 to 8 hours will prevent ongoing dehydration.
Source Control
Once the patient is resuscitated, attention should shift to establishing source control. As previously mentioned, a contrastenhanced CT scan is instrumental in identifying un-drained collections and abscesses. In post-operative patients, source control is most likely accomplished by interventional radiology. However, in the face of inaccessible collections or uncontrolled intra-abdominal sepsis, an operation may be unavoidable. Broad spectrum, empiric intra venous antibiotics should not be used routinely to treat ECF unless there is evidence of intra-abdominal collections or wound infection with an associated cellulitis. Whenever possible, antibiotics should be targeted toward specific culture data and limited to no more than 2 weeks.
Effluent Management
The management of success effluent can have a significant impact
on volume status, electrolyte balance, nutrition, and skin integrity.
Adjunct medical management of ECF effluent has traditionally
focused on two main areas: acid neutralization and volume reduction.
The use of proton pump inhibitors can accomplish both goals and the
dose should be titrated until the effluent’s pH is greater than 6 and the
volume of output is less than 1L/day [16]. Although psyllium can be
extremely useful in bulking the effluent and increasing transit time,
antimotility and antisecretory agents including loperamide, atropinediphenoxylate,
codeine, tincture of opium and even methadone, are
the mainstay of volume reduction. Given its role as the universal
gastrointestinal hormone inhibitor, a great deal of research has been
spent studying the effects of exogenous somatostatin on patients with
ECF. Despite a significant number of randomized controlled trials,
significant conclusions are difficult to elucidate secondary to small
sample size and inconsistent study design [7]. Octreotide, a longer
lasting somatostatin analog, has also been investigated as an ECF
adjunct with promising results. Octreotide 100 mcg three times [25]
daily may reduce effluent volume and has been shown to decrease
time to spontaneous closure in some studies [7,22]. As such, a trial
of octreotide therapy for 72 h with close fistula output monitoring
may be warranted, particularly in cases where other therapies have
failed to decrease output volume [20]. Common side effects include
hyperglycemia, headaches and cholelithiasis.
While the data is clear that low output fistulas have an increased
rate of spontaneous closure, it is less clear that decreasing the volume
of output will improve fistula closure. Nonetheless, decreasing
the volume of output can significantly ease the burden of fistula
management and positively impact patient wellbeing.
Wound Care
Perhaps one of the more challenging and resource demanding
aspects of ECF management is local control of effluent. Success
enteric we can rapidly breakdown skin and cause irritation that
is difficult to treat. As such, appropriate skin management with
barrier creams, moisture barriers, pouching appliances and suction
devices are paramount [21]. High output fistulas, in particular,
often require an intricate network of drains, pouches, and suction to
adequately control effluent. Highly specialized wound care teams and
enterostomal therapists are invaluable for both wound management
and patient comfort [22].
With the ubiquitous use of negative pressure therapy (NPT) for
the management of wounds and open abdomens, it is not surprising
that NPT has become part of the surgical armamentarium to deal with
ECF. In the largest series to date, Wainstein et al. [26] reported their
experience with 91 patients with high output fistulas treated with
negative pressure ranging from -350 to -600 mmHg. In addition to
significantly decreasing effluent output from an average of 1400 cc/day
to 138 cc/day, spontaneous closure was achieved in 46% of patients.
In 2016, Misky et al. [27] published a meta-analysis investigating the
use of NPT in ECF. Using ten retrospective studies with a total of 151
patients, these authors found that the median rate of spontaneous was
closure 65% (ranging from 7%-100%) with a median time to closure
of 58 days. These investigators also reported a new fistula rate of 4.4%
- a known complication that has given some practitioners pause.
While more investigation is clearly necessary to determine if NPT
can definitively improve ECF closure rates or decrease the time to
spontaneous closure, the positive benefits of these dressings should
not be overlooked. At the very least, this technology may make the
management of ECF easier by controlling effluent, protecting skin,
and decreasing frequency of dressing changes.
Table 3
Nutrition
Assessment
The nutritional status of an ECF patient is of paramount
importance. Prior to the advent of peptide-based enteral formulas
and total parenteral nutrition (TPN), retrospective historical series
highlight the burden of malnutrition in ECF patients. In a series of
157 patients in the 1950s and 1960s, almost 75% of patients with
proximal fistulas were malnourished. Similar results were reported by
Chapman et al. [23] who found that only a small minority of patients
had optimal nutrition; and that once nutrition was improved, ECF
related mortality plummeted from 55% to 12-16%.
A complete nutritional assessment should be performed on every
patient with an ECF. Although the Harris-Benedict equation provides
a good starting place for calculating nutritional requirements, it is
widely accepted that ECF patients are catabolic and hyper metabolic.
In general, these patients require increased caloric support with at
least 25-30 kcal/kg/day in carbohydrates and fat and 1.5 to 2 grams of
protein/kg/day [22,28].
Nutritional status and progress should be reassessed on a regular
basis. In addition to daily weights, regular nutrition labs such as,
albumin, prealbum in, transferring and CRP should be followed.
Preoperative albumin, in particular, has been shown to be the
strongest predictor of mortality and morbidity following general
surgery [29] and hypoalbuminemia remains a significant predictor
of poor outcomes in ECF patients [29,30]. Perhaps the most studied
protein in ECF, however, is transferrin and levels greater than 140
have been associated with increased spontaneous closure rates and
reduced mortality [28]. Although nitrogen balance can be a helpful
tool, this standard measure can be difficult in patients with high
output fistulas. Additional tests such as indirect calorimetry may be
considered in more complex cases that do not respond to standard
care.
Parenteral vs. enteral nutrition
Parenteral nutrition has played a critical role in the management
of ECF with its use being firmly established by Dudrick in 1969.
In his sentinel article describing the optimal management of TPN,
Dudrick concluded that: “with the exclusive use of parenteral hyper
alimentation, weight gain, positive nitrogen balance, growth and
development [was] regularly achieved” [31]. Since then, TPN has
been universally adopted as a means of providing nutrition while
promoting “bowel rest” and simplifying effluent management.
While TPN reduce GI secretions by 30%-50%, thereby reducing
the incidence of dehydration and electrolyte imbalances, there are
no randomized studies addressing the impact of TPN on anabolic
conversion, spontaneous closure rates, or mortality. Nonetheless,
TPN remains a popular long-term treatment modality despite serious
complications including infection and liver dysfunction.
More recently, however, the use of enteral nutrition in ECF has
been gaining traction. The use of enteral nutrition has a number of
benefits including decreased cost, fewer infections, and improved
immunologic function when compared to TPN [32]. Moreover, even
providing as little as 20% of calories via an enteral route can help to maintain gut flora and decrease bacterial translocation [22].
Several series have reported surprising success with the enteral
feeding in ECF patients. In a retrospective series by Li et al. [2] 86.4%
of 1168 patients were managed effectively with enteral support.
Enteral nutrition can even be successfully used in the setting of
high output fistula when combined with of elemental formulas, anti
motility agents and fiber bulking agents. Ina series of 335 patients with
high output fistulas, Levy et al. were able to successfully achieve TPN
independence in 85% of their cohort using enteral feeds. Interestingly,
despite enteral nutrition and the lack of “bowel rest”, mortality and
rates of spontaneous closure were similar to prior series.
In the setting of a high output ECF with a distal mucocutaneous
limb, fistuloclysis can be an important adjunct to standard enteral
feeding. The practice of fistuloclysis originated from the practice of
refeeding of chyme in neonates and can refer to either the refeeding of
success or supplementing additional enteral tube feeds via a catheter
placed in the distal limb of an ECF. Teubner et al. [33] first described
fistuloclysis as a therapy for ECF in 2004 in a case series of twelve
patients with proximal fistulae. Since then, a number of case reports
and small series have demonstrated the nutritional efficacy of this
technique [34]. When compared to a matched cohort of patients
receiving TEN without fistuloclysis, patients with high output fistulas
treated with fistuloclysis had significantly improved liver function
and nutritional parameters [35]. Fistuloclysis may be particularly
helpful in patients that develop TPN-related complications including
infections, venous access issues or hepatic failure. Complications
however, can occur and may include obstruction should the peristaltic
activity of the small bowel advance the fistula catheter distally into the
small bowel.
Vitamins and minerals are often depleted in ECF patients.
Deficiencies in fat-soluble vitamins are common, whereas deficiencies
in water-soluble vitamins are rare unless the fistula is within the
proximal jejunum. Moreover, because vitamin B12 is only absorbed
in the terminal 50 to 60 cm of the ileum, injectable vitamin B12 may
be required in many ECF patients. Magnesium is frequently wasted
in high output fistulas and should be replete with either intravenous
magnesium sulfate or oral magnesium chloride for improved enteral
absorption. Finally, high dose repletion of both zinc and vitamin
C has been recommended [22]. While Omega-3 and fish oil have
been studied in the intensive care setting with some benefit, these
supplements have never been evaluated in ECF patients and are not
routinely used [36].
Operative considerations
The rate of spontaneous ECF closure varies between 15 and
75% in the literature. This wide range of closure rates likely reflects
differences in patient populations and institutional practice
patterns [1,5,11,36,37]. Fistulas are significantly less likely to close
spontaneously beyond 4 weeks with only 10% of fistulas closing after
two months [22,38]. Because the rates of spontaneous ECF closure
are low, surgical intervention is often the only definitive management
strategy.
The decision to operate on an ECF, however, should not be taken
lightly as recurrence rates following ECF takedown range from 13
to 34% [20]. Although the principles of surgical intervention for
ECF involve many of the core tenets of general surgery, the time to
operation is perhaps the most important factor to consider. Fazio
et al demonstrated the importance of expectant management when
caring for ECFs patients. When surgery occurred less than 6 weeks
from the time of ECF formation, mortality exceeded 20%. By delaying
operative more than 6 weeks, however, mortality decreased to 11%.
Furthermore, early operation possibly results in increased recurrence
risk. In a series by Lynch et al., patients operated on between 2 and 12
weeks ECF formation had a recurrence rate of 28%, whereas a delay
longer than 12 weeks decreased this rate to 15% [39]. Table 3 shows
the length of time expert authors suggest waiting prior to considering
operative intervention for a non-healing ECF.
Operative management should only be pursued with the full
investment and understanding of both patient and surgeon. ECF
closures are long, technically challenging cases with the risk of
significant complications. Operations often require complete
mobilization of the bowel from the Ligament of Treitz to the rectum.
Given that 36% of recurrent fistulas are the result of injuries created
during the ECF closure operation, great care should be taken
throughout the lysis of adhesions to avoid any inadvertent injury to
healthy bowel [40]. Management of the fistulous bowel should always
entail a bowel resection and primary an astomosis. Over sewing or
wedge resection of the fistula invariably results in a higher recurrence
rate (36% vs. 16%) [39,41].
Conclusion
Entero cutaneous fistula is one of the most challenging complications facing the general surgeon. Utilizing a careful, systematic and patient-centered approach will help to maximize successful clinical outcomes. Despite the consistency of the tenets of ECF management across patients, each fistula is unique and its management is defined in large part by its output as well as its location and patient co-morbidities. Surgeon-specific and institutional experience underscores the importance of delayed intervention. Although surgeons and patients alike may have a strong desire to proceed with surgical closure, patients are best served by at least 6 months of non operative management. Ultimately, the long-term care of ECF patients requires, multi-disciplinary often coordinated by the surgeon.
References
- Hollington P, Mawdsley J, Lim W, Gabe SM, Forbes A, Windsor AJ. An 11-year experience of enterocutaneous fistula. Br J Surg. 2004; 91:1646-51.
- Li J, Ren J, Zhu W, Yin L, Han J. Management of enterocutaneous fistulas: 30-year clinical experience. Chin Med J (Engl). 2003;116:171-5.
- McIntyre PB, Ritchie JK, Hawley PR, Bartram CI, Lennard‐Jones JE. Management of enterocutaneous fistulas: A review of 132 cases. Br J Surg. 1984;71:293-6.
- Soeters PB, Ebeid AM, Fischer JE. Review of 404 patients with gastrointestinal fistulas. Impact of parenteral nutrition. Ann Surg. 1979;190:189-202.
- Rahbour G, Gabe SM, Ullah MR, Thomas GP, Al-Hassi HO, Yassin NA, et al. Seven-year experience of enterocutaneous fistula with univariate and multivariate analysis of factors associated with healing: development of a validated scoring system. Colorectal Dis. 2013;15:1162-70.
- Gonzalez-Pinto I, Gonzalez EM. Optimising the treatment of upper gastrointestinal fistulae. Gut. 2001;49 Suppl 4:iv22-31.
- Lloyd DA, Gabe SM, Windsor AC. Nutrition and management of enterocutaneous fistula. Br J Surg. 2006;93:1045-55.
- Berry SM, Fischer JE. Classification and pathophysiology of enterocutaneous fistulas. Surg Clin North Am. 1996;76:1009-18.
- Fischer JE. The pathophysiology of enterocutaneous fistulas. World J Surg. 1983;7:446-50.
- Draus JM, Jr., Huss SA, Harty NJ, Cheadle WG, Larson GM. Enterocutaneous fistula: are treatments improving? Surgery. 2006;140:570-6.
- Martinez JL, Luque-de-Leon E, Mier J, Blanco-Benavides R, Robledo F. Systematic management of postoperative enterocutaneous fistulas: factors related to outcomes. World J Surg. 2008;3:436-43.
- Misky A, Hotouras A, Ribas Y, Ramar S, Bhan C. A systematic literature review on the use of vacuum assisted closure for enterocutaneous fistula. Colorectal Dis. 2016;18:846-51.
- Campos AC, Andrade DF, Campos GM, Matias JE, Coelho JC. A multivariate model to determine prognostic factors in gastrointestinal fistulas. J Am Coll Surg. 1999;188:483-90.
- Levy E, Frileux P, Cugnenc PH, Honiger J, Ollivier JM, Parc R. High-output external fistulae of the small bowel: management with continuous enteral nutrition. Br J Surg. 1989;76:676-9.
- Lundy JB, Fischer JE. Historical perspectives in the care of patients with enterocutaneous fistula. Clin Colon Rectal Surg. 2010;23:133-41.
- Bleier JI, Hedrick T. Metabolic support of the enterocutaneous fistula patient. Clin Colon Rectal Surg. 2010;23:142-8.
- Teixeira PGR, Inaba K, Dubose J, Salim A, Brown C, Rhee P, et al. Enterocutaneous fistula complicating trauma laparotomy: A major resource burden. Am Surg. 2009;75:30-2.
- Irving M, White R, Tresadern J. Three years' experience with an intestinal failure unit. Ann R Coll Surg Engl. 1985;67:2-5.
- Schecter WP, Hirshberg A, Chang DS, Harris HW, Napolitano LM, Wexner SD, et al. Enteric fistulas: principles of management. J Am Coll Surg. 2009;209:484-91.
- Gribovskaja-Rupp I, Melton GB. Enterocutaneous Fistula: Proven Strategies and Updates. Clin Colon Rectal Surg. 2016;29:130-7.
- McNaughton V, Canadian Association for Enterostomal Therapy ECFBPRP, Brown J, Hoeflok J, Martins L, McNaughton V, et al. Summary of best practice recommendations for management of enterocutaneous fistulae from the Canadian Association for Enterostomal Therapy ECF Best Practice Recommendations Panel. J Wound Ostomy Continence Nurs. 2010;37:173-84.
- Evenson AR, Fischer JE. Current management of enterocutaneous fistula. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2006;10:455-64.
- Chapman R, Foran R, Dunphy JE. Management of Intestinal Fistulas. Am J Surg. 1964;108:157-64.
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580-637.
- Hesse U, Ysebaert D, de Hemptinne B. Role of somatostatin-14 and its analogues in the management of gastrointestinal fistulae: clinical data. Gut. 2001;49:iv11-21.
- Wainstein DE, Fernandez E, Gonzalez D, Chara O, Berkowski D. Treatment of high-output enterocutaneous fistulas with a vacuum-compaction device. A ten-year experience. World J Surg. 2008;32:430-5.
- Edmunds LH, Jr., Williams GM, Welch CE. External fistulas arising from the gastro-intestinal tract. Ann Surg. 1960;152:445-71.
- Polk TM, Schwab CW. Metabolic and nutritional support of the enterocutaneous fistula patient: a three-phase approach. World J Surg. 2012;36:524-33.
- Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri SF. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg. 1999;134:36-42.
- Martinez JL, Luque-de-Leon E, Ballinas-Oseguera G, Mendez JD, Juarez-Oropeza MA, Roman-Ramos R. Factors predictive of recurrence and mortality after surgical repair of enterocutaneous fistula. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2012;16:156-63; 63-4.
- Dudrick SJ, Wilmore DW, Vars HM, Rhoads JE. Can intravenous feeding as the sole means of nutrition support growth in the child and restore weight loss in an adult? An affirmative answer. Ann Surg. 1969;169:974-84.
- Elke G, van Zanten AR, Lemieux M, McCall M, Jeejeebhoy KN, Kott M, et al. Enteral versus parenteral nutrition in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials. Crit Care. 2016;20:117.
- Teubner A, Morrison K, Ravishankar HR, Anderson ID, Scott NA, Carlson GL. Fistuloclysis can successfully replace parenteral feeding in the nutritional support of patients with enterocutaneous fistula. British Journal of Surgery. 2004;91:625-31.
- Ham M, Horton K, Kaunitz J. Fistuloclysis: case report and literature review. Nutr Clin Pract. 2007;22:553-7.
- Wu Y, Ren J, Wang G, Zhou B, Ding C, Gu G, et al. Fistuloclysis improves liver function and nutritional status in patients with high-output upper enteric fistula. Gastroenterol Res Pract. 2014;2014.
- Schecter WP. Management of enterocutaneous fistulas. Surg Clin North Am. 2011;91:481-91.
- Haffejee AA. Surgical management of high output enterocutaneous fistulae: a 24-year experience. Curr Opin Clin Nutr Metab Care. 2004;7:309-16.
- Ross H. Operative surgery for enterocutaneous fistula. Clin Colon Rectal Surg. 2010;23:190-4.
- Lynch AC, Delaney CP, Senagore AJ, Connor JT, Remzi FH, Fazio VW. Clinical outcome and factors predictive of recurrence after enterocutaneous fistula surgery. Ann Surg. 2004;240:825-31.
- Runstrom B, Hallbook O, Nystrom PO, Sjodahl R, Olaison G. Outcome of 132 consecutive reconstructive operations for intestinal fistula--staged operation without primary anastomosis improved outcome in retrospective analysis. Scand J Surg. 2013;102:152-7.
- Reber HA, Roberts C, Way LW, Dunphy JE. Management of external gastrointestinal fistulas. Ann Surg. 1978;188:460-7.