Research Article
Application of Octacalcium Phosphate and Collagen Composite for Bone Augmentation with Sinus Floor Elevation in Humans
Tadashi Kawai1*, Tetsu Takahashi1, Keiko Matsui1, Yushi Ezoe1, Fumihiko Kajii BS2,3, Yoshinaka Shimizu1, Shigeto Koyama4, Hiroyuki Kumamoto1, Osamu Suzuki2and Shinji Kamakura3
1Department of Oral Medicine and Surgery, Tohoku University Graduate School of Dentistry, Japan,
2Department of Oral Medicine and Surgery, Toyobo Co Ltd, Japan
3Department of Regenerative and Biomedical Engineering, Tohoku University Graduate School of Biomedical
Engineering, Japan
4Department of Oral Medicine and Surgery, Tohoku University Hospital, Japan
*Corresponding author: Tadashi Kawai, Department of Oral Medicine and Surgery, Division of Oral and Maxillofacial Surgery, Tohoku University Graduate School of Dentistry, 4-1, Seiryo-machi, Aoba-ku, Sendai, Miyagi, 980-8575, Japan
Published: 18 Sep 2018
Cite this article as: Kawai T, Takahashi T, Matsui K,
Ezoe Y, Fumihiko Kajii BS, Shimizu
Y, et al. Application of Octacalcium
Phosphate and Collagen Composite for
Bone Augmentation with Sinus Floor
Elevation in Humans. Clin Surg. 2018;
3: 2112.
Abstract
Octacalcium Phosphate (OCP) and collagen composite (OCP/Col) was recognized as a highly
osteoconductive bone substitute material based on in vitro and in vivo studies. In this study, OCP/
Col was applied for bone augmentation with sinus floor elevation as a clinical trial. This study
evaluated the progress of bone formation via OCP/Col and dental implant treatment. OCP/Col was
applied in 3 patients, and these patients were evaluated subsequent bone formation radio graphically
and examined the newly formed bone histological and via Fourier transform infrared spectroscopy.
Radio graphical examination showed that OCP/Col converted to hard tissue with radiopacity within
6 months of implantation. Histological examination confirmed normal bone tissue in a specimen
and FTIR revealed characteristics of bone-like apatite. There was no problem after the treatment of
the dental implant. This study suggested that OCP/Col could be a bone substitute material for bone
augmentation procedures involving sinus floor elevation.
Keywords: Bone substitute material; Bone augmentation; Dental implant treatment
Introduction
Various materials have been used as bone substitutes in cases requiring bone reconstruction
[1-4]. Autologous bone grafting is the most desirable approach for bone defect reconstruction, due
to its high osteoconductivity [5]. It has certain disadvantages however, including limited availability,
and the morbidity associated with harvesting bone from a secondary operative site [6-8]. Calcium
phosphate is used in clinical settings as a bone substitute material [1,9-11]. Some calcium phosphate
products have also been used clinically as bone substitutes in dental implant surgery requiring bone
augmentation [3,12-15].
Synthetic Octacalcium Phosphate (OCP) has become recognized as a highly osteoconductive
bone substitute material based on in vitro and in vivo studies [16-18]. OCP has higher solubility
than Hydroxapatite (HA) and beta tricalcium phosphate and is therefore more resorbable than both
of those materials in vivo [19]. It has been reported that OCP combined with collagen (OCP/Col)
facilitates bone regeneration more effectively than OCP alone [20,21], and the osteoconductivity
of OCP/Col has been demonstrated in critical-sized bone defects in dogs [22-25]. Previous studies
have demonstrated the effects of OCP/Col on bone regeneration small bone defects in humans,
and confirmed healing at the sites of the defects [26-28]. However, to date bone augmentation
using OCP/Col at sites of bone atrophy has not been investigated. There is a report of the clinical
use of OCP granules to fill the space created during sinus floor elevation, but the success of the
dental implant treatment was not described in that report [29]. The present study was designed to
investigate whether OCP/Col could be used as a bone substitute material in bone augmentation
for dental implant treatment. The current study reported the first application of OCP/Col for bone
augmentation with sinus floor elevation in patients with atrophic maxilla, and evaluated their
progress until the final prostheses were fitted. Furthermore, the newly formed bone was analyzed via
histological and via Fourier Transform Infrared Spectroscopy (FTIR) for the first time in humans.
Figure 1
Figure 1
Photograph of OCP/Col discs, which are 9 mm in diameter and
1.5 mm thick. The weight percentage of OCP granules in OCP/Col was 77%.
OCP/Col has almost 92% porosity and bimodal peaks of 48 μm (main pores)
and 0.3 μm (minor pores), and its specific surface area (SSA) was 17.8 m2/g.
Figure 2
Figure 2
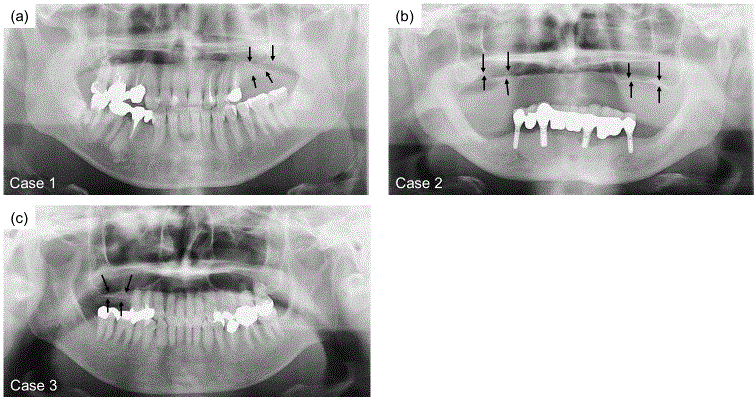
X-ray images of maxillary atrophic patients. (a) Case 1, the right
maxilla. (b) Case 2, bilateral maxilla. (c) Case 3, the left maxilla. Arrows show
the height of the alveolar crest relative to the sinus floor. The thickness of
bone from the alveolar crest to the sinus floor in each case was 1 mm to 5
mm. Bone augmentation was necessary for dental implant treatment.
Materials and Methods
Preparation of OCP/Col
OCP was prepared by mixing calcium acetate hydrate solution and
sodium phosphate monobasic solution as described previously [16].
The precipitates were washed several times with filter-sterilized water,
and then lyophilized. Sieved OCP granules (particle sizes 300 μm to
500 μm) obtained from the dried OCP were sterilized via heating at
120°C for 2hr. A previous study showed that such heating does not
affect physical properties such as the crystalline structure or specific
surface area of OCP granules, although increasing the temperature
above 100°C induced collapse of the OCP structure due to dehydration
[30]. Powder X-ray Diffraction (XRD) patterns of the processed
OCP were recorded via step-scanning at 0.05-degree intervals from
3 to 60 degrees, with Cu Kα X-rays on a diffract meter (Mini Flex;
Rigaku Electrical Co, Tokyo, Japan) at 30 kV and 15 mA. The range
of 2θ included the primary peak (100) reflection of OCP, which
was approximately 4.7 degrees. Collagen was prepared from NMP
collagen PS (Nippon Meat Packers, Tsukuba, Ibaraki, Japan) and a
lyophilized powder was prepared from pepsin-digested atelocollagen
isolated from porcine dermis. NMP collagen PS was dissolved in
filter-sterilized water and adjusted to a final concentration of 3%
and pH 7.4 OCP/Col was prepared from NMP collagen PS and OCP
granules. OCP was added to the concentrated collagen and mixed.
The weight percentage of OCP in OCP/Col was 77%. This OCP/
Col mixture was then lyophilized and discs were moulded (9-mm
diameter, 1.5-mm thickness) (Figure 1). The molded OCP/Col was
dehydrothermally treated (150°C, 24 hr) in a DP32 Vacuum Drying
Oven (Yamato Scientific, Tokyo, Japan). The clinical batches were
prepared aseptically. Five pieces of the molded OCP/Col were placed
in a sterilized micro centrifuge tube, and the tube containing OCP/
Col was then packed into an aluminum pouch. The packed OCP/Col
was subsequently sterilized using electron beam irradiation (22 kGy)
to render it ready for use. After sterilization, the XRD pattern derived
from OCP/Col included a collapsed and reduced primary (100) peak
with a shift from 4.7 to 5.3 degrees at 2θ, as previously reported
[20]. OCP/Col has almost 92% porosity and bimodal peaks of 48
μm (main pores) and 0.3 μm (minor pores), and its specific surface
area (SSA) was 17.8 m2/g, as previously reported [31]. The OCP/Col
before implantation was scanned with a Microfocus X-ray CT System
(Scan Xmate-E090; Comscantecno Co., Ltd., Kanagawa, Japan) with
settings of 90 kV and 0.1 mA, and the image data were calculated using
a three-dimensional (3D) image analysis system (TRI/3D-Bon; Ratoc
System Engineering, Tokyo, Japan) as previously described [26].
OCP/Col itself has little radiopacity, and the Computed Tomography
(CT) values of the OCP/Col before implantation ranged from 130 to
140 Hounsfield Units (HU) [26].
Design of the clinical trial
The current study was a part of the ‘Bone Augmentation by Octacalcium Phosphate Collagen Composite’ clinical trial, and a
part of the ‘Prospective, Multi-center, Single-arm Study of OCP/Col
for Guided Bone Regeneration’ clinical trial, which were registered
with the Medical Information Network in Japan (UMIN; registration
numbers JPRN-UMIN000015852 and JPRN-UMIN000018192,
respectively). The protocol of the former trial was approved by the
Research Ethics Committee of Tohoku University Graduate School
of Dentistry under reference number [25,26]. This clinical trial was
a single-arm non-randomized intervention study. The principal
investigator and promoter was Dr. Tadashi Kawai (D.D.S, Ph.D),
and this study was performed at the Dept. of Oral and Maxillofacial
Surgery, in Tohoku University Hospital, in Sendai, Japan. The protocol
of the latter clinical trial was approved by the Institutional Review
Board of the Pharmaceuticals and Medical Devices Agency in Japan
under reference number OCTC-14001. The sponsor of that clinical
trial was Toyobo CO, LTD, and it was a single-arm non-randomized
intervention study performed at 9 hospitals in Japan. The aim of the
first clinical evaluation in these clinical trials was to assess the safety
of the use of OCP/Col to fill bone defects after sinus floor elevation
via clinical examinations and analysis of adverse events. The second
clinical evaluation was focused on bone regeneration assessment via
radiographic and histological examinations. All patients included in
the current study provided informed written consent.
Clinical cases
The present study describes the treatment of 3 cases of atrophic
posterior maxilla at Tohoku University Hospital. A 41-year-old male
Japanese patient (case 1), a 68-year-old male Japanese patient (case
2) and a 54-year-old female Japanese patient (case 3) with nothing
noteworthy in their medical records were referred to our hospital for
dental implant treatment in the maxilla region. As dental implant
placement was impossible because bones in their maxillae were very
thin, bone augmentation with a sinus floor elevation technique was
necessary before dental implant placement. The respective operative
sites of cases 1, 2 and 3 were the left maxillary sinus, bilateral maxillary
sinuses and right maxillary sinus (Figure 2a-2c). We performed sinus
floor elevation under local Anesthesia in case 1 and 3, and general
anaesthesia in case 2. Gingiva and periosteum were ablated and an
oval-shaped groove was formed in the lateral wall of the maxillary
sinus. Sinus membrane was ablated from the sinus floor, and the
membrane was lifted upward with the oval-shaped bone of the
lateral wall region (Figure 3a). There was no perforation of the sinus
membrane in any of the cases. OCP/Col discs were placed in the space
(Figure 3b), and then the gingiva and periosteum were repositioned
and sutured.
Clinical and radiographic examination
Clinical examinations involved observation of general and local
conditions including inflammatory symptoms at the operative sites.
In addition, laboratory examination was carried out. Radiographic
and clinical examinations were performed prior to the operations,
and 1 day, 7 days, and 1, 3 and 6 months after OCP/Col implantation.
CT scans were performed before the operation and 3 and 6
months after OCP/Col implantation. Coronal scans of the surgical
site were obtained using a CT scanner (Somatom Definition flash,
Siemens, Germany) at a tube current of 35 mA, a tube voltage of
120 kV and a slice thickness of 0.6 mm. The scanner was calibrated
every working day, using a water phantom. The regions of interest
(ROIs) were defined at the center of the augmented region. Each
ROI was circular and 5 mm in diameter. The HU values of the ROI
were measured using software (We View Open-Pacs series, Hitachi
Medical Corp, Tokyo, Japan) and all values were reported as mean ±
Standard Deviation (SD).
Dental implant placement
After confirmation of bone augmentation by CT at 6 months,
dental implant placement was performed under local anaesthesia.
Gingiva and periosteum were ablated and dental implant bodies were
inserted into the maxillary region. The Dental implant systems were
the Astra Tech Implant System, OsseoSpeedTM; Dentsply Sirona Inc.,
Tokyo, Japan or the Straumann® Bone Level Implant; Straumann
Japan Inc, Tokyo, Japan.
Histological examination and FTIR analysis
At the time of the dental implant placement operation, samples
of newly formed bone were collected from sites where dental
implants were going to be inserted, using a trephine bar with a
diameter of 2.4 mm in each case. The samples were fixed with 4%
para formaldehyde in 0.1 M phosphate-buffered saline, pH 7.4 for a
few days, and decalcified in 10% ethylenediaminetetraacetic acid in
0.01 M phosphate buffer, pH 7.4, for 2-4 weeks at 4°C. The samples
were dehydrated in a graded series of ethanol concentrations and
embedded in paraffin. Each sample was then sectioned at a thickness
of 5 mm. The sections were stained with hematoxylin and eosin, and
photographs were taken with a photomicroscope (Leica DFC290 HD,
Leica Microsystems Japan, Tokyo, Japan). One sample of case 1 was
immediately washed in deionized water, lyophilized and reduced to
powder. The FTIR spectrum was obtained by a JASCO FT/IR-6300
(JASCO, Tokyo, Japan), with the sample diluted with KBr over a
range of 4000–400 cm-1 with a 4-cm-1 resolution.
Figure 3
Figure 3
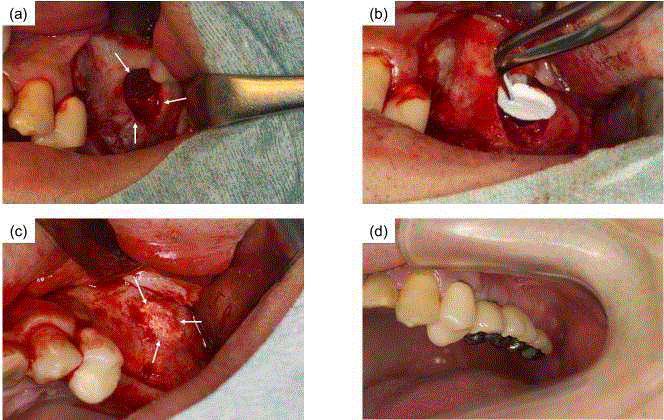
Photographs of sinus floor elevation in case 1 (a) Gingiva and
periosteums were ablated and an oval-shaped groove was formed in the
lateral wall of the maxillary sinus. A space was created from the lateral sinus
wall region (arrows). (b) There was no perforation of the sinus membrane.
OCP/Col discs were implanted into the space. (c) Hard tissue was confirmed
at the lateral sinus wall region at 6-months after OCP/Col implantation
(arrows). There were no abnormalities or any inflammation at the time of the
second dental implant surgery. (d) The final prosthesis was set.
Figure 4
Figure 4
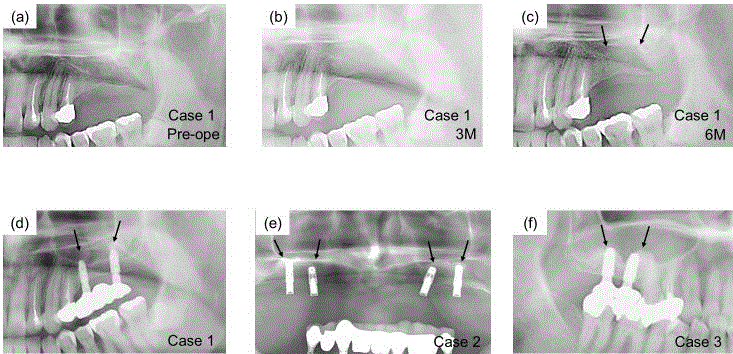
X-ray images after OCP/Col implantation. (a) Immediately after
OCP/Col implantation in case 1. There was little radiopacity in the OCP/Col
implantation region. (b) Three months after dental implant placement in case
1. The sinus floor had become unclear. (c) Six months after dental implant
placement in case 1. Sinus floor augmentation was observed. (d) Twelve
months after dental implant placement in case 1 (e) Twelve months after
dental implant placement in case 2 (f) Twelve months after dental implant
placement in case 3. Arrows show the elevated sinus floor. In each case, the
implant body was stable without inflammatory findings.
Figure 5
Figure 5
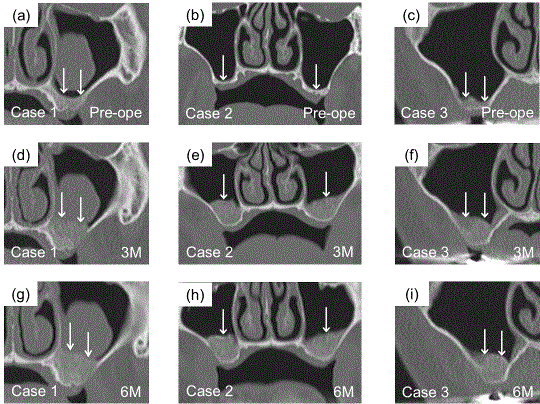
CT images of the coronal plane at 3 and 6 months after OCP/
Col implantation. (a-c) Before sinus floor elevation (original sinus) in cases
1, 2 and 3. (d-f) At 3 months after the operation. OCP/Col was detected as
hard tissue with radiopacity. (g-i) At 6 months after the operation. The shape
of radiopacity was stable in each case, and the radiopacity had increased
relative to that at 3 months. The heights of hard tissue at the area of implanted
OCP/Col were almost the same as at 3 months, with no signs of absorption.
There was a mucous cyst in the sinus in case 1 before OCP/Col implantation.
Figure 6
Figure 6
Magnified view of the specimens stained by hematoxylin and eosin.
(a) Case 1 (b) Case 2 (c) Case 3. Newly formed bone was observed around
remaining granules of OCP/Col. Neither scar tissue nor inflammatory cell
infiltration were seen. Bar=200 μm* =remaining implants. B=newly formed
bone.
Table 1
Table 1
CT values in each case at 3 and 6 months after OCP/Col implantation. All values represent mean ± SD. CT value of the OCP/Col before implantation ranged
from 130 HU to 140 HU. CT values increased to approximately 300 HU to 400 HU that was almost the same as cancellous bone.
Results
Clinical examination
Although swelling and redness at the operative region were
observed after several days, the severity of these symptoms was
approximately equal to that seen after a normal operation, and
disappeared within 7 days in all cases. Laboratory examination
indicated increases in C-Reactive Protein (CRP) or White Blood Cells
(WBCs) the day after implantation, but these parameters recovered
normal levels within 7 days in all cases. The wound healed well and
there was no outflow of OCP/Col. Postoperative wound healing was
satisfactory, and there were no postoperative infections or allergic
reactions at the operative site during the observation period. At the
time of dental implant placement surgery, hard tissue was confirmed
in the lateral window region (Figure 3c). There were no abnormalities
or any inflammation at the time of the second dental implant surgery
for the exchange of abutments approximately 6 months later. After
that, a final prosthesis was placed and occlusion was reconstructed
(Figure 3d). Each patient had a different type of prosthesis fitted, and
the three types were an implant bridge, an implant over denture and
an implant crown.
Radiographic examination
In radiographic examination, the thickness of bone from the
alveolar crest to the sinus floor in the left maxillary region in case
1 was almost 5 mm before OCP/Col implantation. In case 2, that
of bilateral maxillaries was 1 mm to 2 mm, and that of case 3 was
5 mm. Radiopacity was not observed at the operated region the day
after OCP/Col implantation in any of the cases, because OCP/Col
has little radiopacity (Figure 4a). However, radiopacity had appeared
at the area of implanted OCP/Col and the sinus floor had become
unclear at 3 months (Figure 4b). At 6 months, the radiopacity had
further increased and was almost equal to that of surrounding bone.
The sinus floor had become clear and was augmented (Figure 4c).
Radiopacity was stable at 12 months after dental implant placement
with the final prosthesis (Figure 4d-4f).
Figures 5a-5c show CT pictures of the 3 cases before OCP/Col
implantation. Mucous cyst was confirmed in the sinus of case 1 before
sinus floor elevation. At 3 months after OCP/Col implantation,
a coronal CT view indicated radiopacity at the area of implanted
OCP/Col in the sinus (Figure 5d-5f). At 6 months, radiopacity had
further increased (Figure 5g-5i). The heights of hard tissue at the
area of implanted OCP/Col were almost the same, with no signs of
absorption. In case 1, the respective CT values of the augmentation
region at 3 and 6 months were 260.59 ± 56.99 and 353.78 ± 78.10.
Those of case 2 were 253.17 ± 63.44 and 317.84 ± 160.89 in the right
maxillary sinus and 278.72 ± 44.62 and 332.70 ± 145.31 in the left
maxillary sinus. Those of case 3 were 178.15 ± 121.64 and 289.85 ±
91.73 (Table 1).
Histological examination and FTIR analysis
Figure 6 shows the alveolar crest biopsy specimens from each
case. Newly formed bone was observed around remaining granules
of OCP/Col (Figure 6a-6b). In case 3, there was no remaining OCP
around bone tissue (Figure 6c). Neither scar tissue nor inflammatory
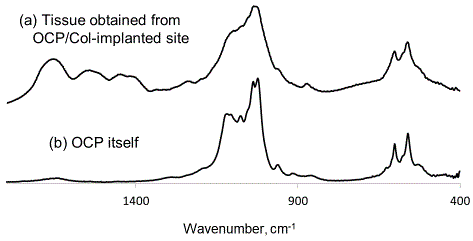
cell infiltration were seen. Figure 7 shows the FTIR spectra of the
specimen of case 1 and OCP itself. The features of FTIR spectra of
the specimen indicate conversion of OCP/Col into HA in the IR
spectral bands around 1030–1130 cm-1. The features of CO32- were confirmed at bands around 870 and 1410–1460 cm-1 in the spectra of
the specimen. The specimen did not exhibit any of the characteristic
features of OCP.
Figure 7
Figure 7
FTIR spectra of (a) tissue obtained from OCP/Col-implanted site,
and (b) OCP itself. IR spectral bands at approximately 1030-1130 cm-1
changed to those of HA in tissue obtained from OCP/Col-implanted site. The
features of CO32- were confirmed at bands at approximately 870 and 1410-
1460 cm-1 in the spectra of the specimen.
Discussion
Various materials are currently used as bone substitute materials
in dental implant treatment and its effectiveness has been reported
[3,14,15], although they are inferior to autologous bone. Many studies
have been performed with the aim of improving osteoconductivity by
combining these materials [32], or incorporating other factors [33]
or mesenchymal stem cells [34]. Today, other groups have reported
superior bone regeneration via the mixing of autologous bone and artificial material [35]. However, the results in this study provide
evidence of a single material, OCP/Col, with high osteoconductivity
and effectiveness for dental implant treatment.
In this study, OCP/Col was applied in conjunction with sinus
floor elevation, in 4 maxillary sinuses of 3 patients. Previous studies
demonstrated that there was no abnormality such as infection or
inflammation in 10 patients in whom OCP/Col was applied as a
treatment for defects caused by cystectomy or tooth extraction [28].
Likewise, the abnormality finding that was apparent to 3 patients in
the current study was not confirmed. Thus, it has been suggested that
OCP/Col is safe for clinical use in humans. OCP/Col consists of crosslinked
collagen, so it was easy to use OCP/Col in the operative region
without it collapsing, and OCP/Col was stable in the space because the
collagen adhered to living tissue well. Therefore, it was considered that
OCP/Col could be applied to a case of small perforation of the sinus
membrane that was a surgical complication of sinus floor elevation
[36]. Usually in such a case, an absorbent membrane sheet product
is used to cover the perforated region, and bone substitute materials
are placed in the created space, or the operation is terminated [37].
However, OCP/Col could cover the perforated region if the size was
small, and function as a bone substitute by itself.
OCP/Col converted to hard tissue over time, as determined radio
graphically. In this study, this phenomenon made it easy to confirm
bone augmentation via dental radiography. At 3 months, radiopacity
was observed at the OCP/Col region and it had become clear by 6
months in all sinuses. Furthermore, there was little change even at
12 months after dental implant placement. CT pictures showed
radiopacity at the OCP/Col region at 3 or 6 months. The heights
of augmentation of each sinus were almost maintained without
absorption, and the border between the host bone of maxilla and
hard tissue formed via OCP/Col became unclear within 6 months.
CT values indicated increasing over time. The CT value of OCP/Col
is approximately 130 HU to 140 HU. At 3 months, the CT values
of each sinus increased to approximately 200 HU to 300 HU, and
furthermore, these values tended to be increased at 6 months, to
approximately 300 HU to 400 HU. The values at 6 months were similar
to that of cancellous bone. The SDs of CT values were substantial at
approximately 100 in each sinus. The inside of the maxilla comprised
cancellous bone that included a sparse part. Therefore, it was thought
that the bone that was newly formed via OCP/Col was similar to the
cancellous bone of the maxilla, as indicated by the SDs of the CT
values in this study.
Previous studies have demonstrated that OCP/Col converted to
normal bone tissue after implantation in rat and dog bone defects
[22]. In the current study, the conversion of OCP/Col to normal
bone tissue was confirmed histological in humans for the first time.
Furthermore, there was no infiltration of inflammatory cells around
bone that was newly formed via OCP/Col. Some remaining granules
in newly formed bone were detected histological. However, the
features of FTIR spectra indicated the conversion of OCP of OCP/Col
into biological apatite, and the characteristic features of OCP were
not apparent. These results demonstrated that normal bone tissue was
formed around remaining granules and OCP of OCP/Col could be
converted to biological apatite without the OCP itself remaining, as
has been observed in prior animal studies [22,23,38].
It has been approximately 12-18 months since final dental
implant placement in the 3 cases, and no substantial adverse events
have occurred. The dental Implant treatment of these 3 cases was
performed to the point of final prosthesis fitting without any problems.
The final prosthesis was different in each case, the three types being
an implant bridge, an implant over denture, and an implant crown,
and there were no problems with any of the prostheses. There was no
secondary operative site for bone grafting due to the use of OCP/Col
instead of autogenous bone, and each case exhibited good progress.
The period of dental implant treatment from bone augmentation to
final prosthesis was almost the same as that associated with the use of
autogenous bone. This study demonstrated that OCP/Col application
for bone augmentation before dental implant placement was effective,
and that it was safe to use OCP/Col in humans. Furthermore, it was
suggested that OCP/Col could be applied to other cases of bone defect
or atrophy in the field of oral and maxillofacial surgery.
Conclusion
The present study suggested that octacalcium phosphate collagen composite could be applied to bone augmentation in conjunction with sinus floor elevation for maxillary atrophic patients, and that octacalcium phosphate collagen composite could be converted to normal bone tissue in humans.
Acknowledgement
This clinical trial (JPRN-UMIN000015852) was conducted as a collaborative investigation with Toyobo. Co, Ltd. Company (project code: J140002441). Another clinical trial (JPRN-UMIN000018192) has also been conducted by the same company.
References
- LeGeros RZ. Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop Relat Res. 2002(395):81-98.
- Jo SH, Kim YK, Choi YH. Histological Evaluation of the Healing Process of Various Bone Graft Materials after Engraftment into the Human Body. Materials (Basel). 2018;11(5).
- Okada T, Kanai T, Tachikawa N, Munakata M, Kasugai S. Histological and Histomorphometrical Determination of the Biogradation of beta-Tricalcium Phosphate Granules in Maxillary Sinus Floor Augmentation: A Prospective Observational Study. Implant Dent. 2017;26(2):275-83.
- Misch CM. Bone Augmentation Using Allogeneic Bone Blocks With Recombinant Bone Morphogenetic Protein-2. Implant Dent. 2017;26(6):826-31.
- Misch CM. Maxillary autogenous bone grafting. Oral maxillofac surg clin of North Am. 2011;23(2):229-38.
- Clavero J, Lundgren S. Ramus or chin grafts for maxillary sinus inlay and local onlay augmentation: comparison of donor site morbidity and complications. Clin implant Dent Relat Res. 2003;5(3):154-60.
- Cricchio G, Lundgren S. Donor site morbidity in two different approaches to anterior iliac crest bone harvesting. Clin implant Dent Relat Res. 2003;5(3):161-9.
- Nkenke E, Neukam FW. Autogenous bone harvesting and grafting in advanced jaw resorption: morbidity, resorption and implant survival. Eur J Oral Implantol. 2014;7:203-17.
- Bucholz RW. Nonallograft osteoconductive bone graft substitutes. Clin Orthop Relat Res. 2002;395(395):44-52.
- Ogose A, Kondo N, Umezu H, Hotta T, Kawashima H, Tokunaga K, et al. Histological assessment in grafts of highly purified beta-tricalcium phosphate (OSferion) in human bones. Biomaterials. 2006;27(8):1542-9.
- Gaasbeek RD, Toonen HG, van Heerwaarden RJ, Buma P. Mechanism of bone incorporation of beta-TCP bone substitute in open wedge tibial osteotomy in patients. Biomaterials. 2005;26(33):6713-9.
- Minami M, Takechi M, Ohta K, Ohta A, Ninomiya Y, Takamoto M, et al. Bone formation and osseointegration with titanium implant using granular- and block-type porous hydroxyapatite ceramics (IP-CHA). Dent Mater J. 2013;32(5):753-60.
- Zijderveld SA, Zerbo IR, van den Bergh JP, Schulten EA, ten Bruggenkate CM. Maxillary sinus floor augmentation using a beta-tricalcium phosphate (Cerasorb) alone compared to autogenous bone grafts. Int J Oral Maxillofac Implants. 2005;20(3):432-40.
- Guarnieri R, Belleggia F, Ippoliti S, DeVilliers P, Stefanelli LV, Di Carlo S, et al. Clinical, Radiographic, and Histologic Evaluation of Maxillary Sinus Lift Procedure Using a Highly Purified Xenogenic Graft (Laddec®). J Oral Maxillofac Res. 2016;7(1):e3.
- Helder MN, van Esterik FAS, Kwehandjaja MD, Ten Bruggenkate CM, Klein-Nulend J, Schulten E. Evaluation of a new biphasic calcium phosphate for maxillary sinus floor elevation: Micro-CT and histomorphometrical analyses. Clin Oral Implants Res. 2018;29(5):488-98.
- Suzuki O, Nakamura M, Miyasaka Y, Kagayama M, Sakurai M. Bone formation on synthetic precursors of hydroxyapatite. Tohoku J Exp Med. 1991;164(1):37-50.
- Suzuki O, Kamakura S, Katagiri T, Nakamura M, Zhao B, Honda Y, et al. Bone formation enhanced by implanted octacalcium phosphate involving conversion into Ca-deficient hydroxyapatite. Biomaterials. 2006;27(13):2671-81.
- Anada T, Kumagai T, Honda Y, Masuda T, Kamijo R, Kamakura S, et al. Dose-dependent osteogenic effect of octacalcium phosphate on mouse bone marrow stromal cells. Tissue Eng Part A. 2008;14(6):965-78.
- Kamakura S, Sasano Y, Shimizu T, Hatori K, Suzuki O, Kagayama M, et al. Implanted octacalcium phosphate is more resorbable than beta-tricalcium phosphate and hydroxyapatite. J Biomed Mater Res. 2002;59(1):29-34.
- Kamakura S, Sasaki K, Honda Y, Anada T, Suzuki O. Octacalcium phosphate combined with collagen orthotopically enhances bone regeneration. J Biomed Mater Res B Appl Biomater. 2006;79(2):210-7.
- Kawai T, Anada T, Honda Y, Kamakura S, Matsui K, Matsui A, et al. Synthetic octacalcium phosphate augments bone regeneration correlated with its content in collagen scaffold. Tissue Eng Part A. 2009;15(1):23-32.
- Kawai T, Matsui K, Iibuchi S, Anada T, Honda Y, Sasaki K, et al. Reconstruction of critical-sized bone defect in dog skull by octacalcium phosphate combined with collagen. Clin Implant Dent Relat Res. 2011;13(2):112-23.
- Iibuchi S, Matsui K, Kawai T, Sasaki K, Suzuki O, Kamakura S, et al. Octacalcium phosphate (OCP) collagen composites enhance bone healing in a dog tooth extraction socket model. Int J Oral Maxillofac Surg. 2010;39(2):161-8.
- Matsui K, Matsui A, Handa T, Kawai T, Suzuki O, Kamakura S, et al. Bone regeneration by octacalcium phosphate collagen composites in a dog alveolar cleft model. Int J Oral Maxillofac Surg. 2010;39(12):1218-25.
- Miura K, Matsui K, Kawai T, Kato Y, Matsui A, Suzuki O, et al. Octacalcium phosphate collagen composites with titanium mesh facilitate alveolar augmentation in canine mandibular bone defects. Int J Oral Maxillofac Surg. 2012;41(9):1161-9.
- Kawai T, Echigo S, Matsui K, Tanuma Y, Takahashi T, Suzuki O, et al. First clinical application of octacalcium phosphate collagen composite in human bone defect. Tissue Eng Part A. 2014;20(7-8):1336-41.
- Kawai T, Suzuki O, Matsui K, Tanuma Y, Takahashi T, Kamakura S. Octacalcium phosphate collagen composite facilitates bone regeneration of large mandibular bone defect in humans. J Tissue Eng Regen Med. 2017;11(5):1641-7.
- Kawai T, Tanuma Y, Matsui K, Suzuki O, Takahashi T, Kamakura S. Clinical safety and efficacy of implantation of octacalcium phosphate collagen composites in tooth extraction sockets and cyst holes. J Tissue Eng. 2016;7:1-8.
- Komlev VS, Barinov SM, Bozo, II, Deev RV, Eremin, II, Fedotov AY, et al. Bioceramics composed of octacalcium phosphate demonstrates enhanced biological behavior. ACS Appl Mater Interfaces. 2014;6(19):16610-20.
- Suzuki O, Nakamura M, Miyasaka Y, Kagayama M, Sakurai M. Maclura pomifera agglutinin-binding glycoconjugates on converted apatite from synthetic octacalcium phosphate implanted into subperiosteal region of mouse calvaria. Bone Miner. 1993;20(2):151-66.
- Suzuki Y, Kamakura S, Honda Y, Anada T, Hatori K, Sasaki K, et al. Appositional bone formation by OCP-collagen composite. J Dent Res. 2009;88(12):1107-12.
- Schwarz F, Mihatovic I, Golubovic V, Hegewald A, Becker J. Influence of two barrier membranes on staged guided bone regeneration and osseointegration of titanium implants in dogs: part 1. Augmentation using bone graft substitutes and autogenous bone. Clin Oral Implants Res. 2012;23(1):83-9.
- Lee JH, Ryu MY, Baek HR, Lee HK, Seo JH, Lee KM, et al. The effects of recombinant human bone morphogenetic protein-2-loaded tricalcium phosphate microsphere-hydrogel composite on the osseointegration of dental implants in minipigs. Artif Organs. 2014;38(2):149-58.
- Zou D, Guo L, Lu J, Zhang X, Wei J, Liu C, et al. Engineering of bone using porous calcium phosphate cement and bone marrow stromal cells for maxillary sinus augmentation with simultaneous implant placement in goats. Tissue Eng Part A. 2012;18(13-14):1464-78.
- Aludden HC, Mordenfeld A, Hallman M, Dahlin C, Jensen T. Lateral ridge augmentation with Bio-Oss alone or Bio-Oss mixed with particulate autogenous bone graft: a systematic review. Int J Oral Maxillofac Surg. 2017;46(8):1030-8.
- Stacchi C, Andolsek F, Berton F, Perinetti G, Navarra CO, Di Lenarda R. Intraoperative Complications During Sinus Floor Elevation with Lateral Approach: A Systematic Review. Int J Oral Maxillofac Implants. 2017;32(3):e107-e18.
- Hernandez-Alfaro F, Torradeflot MM, Marti C. Prevalence and management of Schneiderian membrane perforations during sinus-lift procedures. Clin Oral Implants Res. 2008;19(1):91-8.
- Tanuma Y, Matsui K, Kawai T, Matsui A, Suzuki O, Kamakura S, et al. Comparison of bone regeneration between octacalcium phosphate/collagen composite and beta-tricalcium phosphate in canine calvarial defect. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(1):9-17.