Research Article
Contribution to Revascularization by Reciprocal Random Skin Flaps
Li Song1,2, Xiu-Fa Tang3, Ping Ji1,4, Li-Na Gao1, Xian-Ming Mo5, Yong Li1,4, Zhao H-W1,4, Zhang F-G1,4* and Xiang X-R1,2*
1Chongqing key Laboratory for Oral Diseases and Biomedical Sciences, Huazhong University of Science and Technology, China
2Department of Periodontology, Chongqing Medical University, China
3Department of Head and Neck Carcinoma, Sichuan University, China
4Department of Oral and Maxillofacial Surgery, Chongqing Medical University, China
5Laboratory of Stem Cell Biology, Sichuan University, China
*Corresponding author: Fu-Gui Zhang, Stomatological Hospital of Chongqing Medical University, 426, N. Songshi Rd, Chongqing 401147, P.R. China
Published: 05 Sep, 2016
Cite this article as: Song L, Tang X-F, Ji P, Gao L-N, Mo X-M, Li Y, et al. Contribution to Revascularization by Reciprocal Random Skin Flaps. Clin Surg. 2016; 1: 1101.
Abstract
Background: The introduction of random skin flaps to plastic surgery has a major impact on wound
closure and the reconstruction of normal and functional anatomical features, but the role of random
skin flaps and the interactions between random skin flaps and recipients during random skin flap
revascularization process remain to be fully elucidated.
Methods: The authors endeavored to establish a reciprocal random skin flap model between green
fluorescent protein (GFP)-transgenic and wild-type (wt) C57BL/6 mice. Blood vessel origination,
growth directions and anastomosis positions were detected by a multitude of detective methods at
postoperative days 3, 7, 14 and 21.
Conclusion: The authors harbored the idea that the random skin flaps played essential roles in
promoting survival and were of vital significance to the ordered process of random skin flap
revascularization by the outgrowth and by the ingrowths of capillary buds of skin flaps through a
unique reciprocal random skin flap model.
Keywords: Transgenic mice; Random skin flap; Revascularization; Physiological mechanism; Therapeutic angiogenesis
Introduction
Random skin flaps are often used in plastic and reconstructive surgery, head and neck surgery,
orthopedic surgery, etc. to repair defects resulting from congenital abnormalities, trauma, or cancer
treatment. However, the potential physiological mechanism of random skin flap revascularization
has been uncertain for hundreds of years. With recent advances in molecular vascular biology,
the fundamental mechanism of flap revascularization has gained increasing interest in the field of
reconstructive surgery.
Early studies of skin graft revascularization mainly regarding free skin graft revascularization
in the late 1800s suggested a direct connection between host and graft vessels, called inosculation.
Before this, free skin graft survival was dependent on imbibition (fluid absorption), followed by a
large number of anastomosis theories [1-4]. Besides inosculation/anastomosis, another principle
of vascularization prevailed. Neovascularization, namely angiogenesis and vasculogenesis, was an
ingrowth of newly formed blood vessels from the wound bed into the graft [4-6]. Concurrently,
Zarem, Capla and Converse et al. [7-9] suggested that the process of vascularization of full-thickness
skin grafts in mice was dominated by inosculation and vascular in growth from the recipient without
a contribution from donor derived cells.
However, few progresses in mechanistic studies have been
reported about pedicle flaps or free flaps. In 1982, Young, using
intravital dye, harbored the idea that the revascularization of pedicle
skin flaps was evident at 3 to 4 days postoperatively at the distal
hypoxic part of viable flaps, and that the whole flap had a collateral
blood supply at 7 to 10 days after surgery [10]. This time frame of
revascularization was similar to the records of wound healing in skin
flaps [11 and 12]. According to the revascularization route by Tsur et al.
[12] the paramount significance of early revascularization arose from
both the wound bed and wound margin.
The interactions between random skin flaps and recipients
remain to be elucidated, although the time frame about pedicle flap
revascularization has been described. Furthermore, what is the role of
random skin flaps in their own survival? The authors adopted a novel
GFP-transgenic rodent model ideal for studying vessel formation
and origination and easily distinguishing growth directions of newly
formed vascular networks from those of preexisting vasculatures as
well as identifying anastomosis sites and time points.
Materials and Methods
Reciprocal random skin flap model
All protocols were approved by the Sichuan University Medical
Center Institutional Animal Care and Use Committee. 40 GFPtransgenic
C57BL/6 mice (State Key Laboratory of Biotherapy,
Sichuan University) and 40 wt C57BL/6 mice (Animal Center for
Medical Experiments, Sichuan University), male, aged 10 to 20 weeks,
were housed under specific pathogen-free conditions and treated
according to NIH guidelines. Intraperitoneal anesthesia of chloral
hydrate (3 mL/kg) was administered because of its long duration
and low death rate. The random skin flap and recipient defect,
approximately 1×1 cm in size, consisted of skin and subcutaneous
fascia, including the panniculus carnosus as previously described [13].
To study the contribution of recipient-derived neovascularization,
caudal recipient defects were brought out on GFP-transgenic mice,
and caudally based random skin flaps were harvested on wt mice
and transplanted to recipient sites. To determine the contribution of
donor-derived neovascularization, cranial recipient sites were created
on wt mice, and cranially based random skin flaps were produced on
GFP-transgenic mice and transferred to recipient sites. All random
skin flaps were sutured to recipient sites with interrupted 5-0 Ethilon
(Ethicon, Inc., Somerville, NJ). Cyclosporine A (20mg/(kg.d);
Novartis Pharma Stein AG, Switzerland) was used for intraperitoneal
injection lasting for one week after surgery. And penicillin was
utilized i.m. at the first three days postoperatively. To assess the flap
survival, random skin flaps were assessed at postoperative days 3, 7,
14 and 21, respectively (n=10 pairs/time point). The mice were then
euthanized by overdose of pentobarbital.
Vascular anastomosis detection
Blood supplies and vascular anastomoses were evaluated by
fluorescent angiography at days 3, 7, 14, and 21 after surgery (n = 5
pairs/time point). Angiography was performed through caudal vein
injection of fluorescite 10% injection (Cardinal Health Manufacturing
Services B.V., Humacao, Puerto Rico) into the GFP-transgenic mice.
Vasculatures of both donor flaps and recipients, and anastomoses
between them were detected by fluorescent angiography using a
Heidelberg retinal tomograph (Heidelberg Engineering GmbH,
Heidelberg, Germany) with exposure conditions of 10 to 16 mA and 24 to 27 kV.
Assessment of origination and sites of neovascularization
To study the contribution of both donor-derived and recipientderived
neovascularization, tissues harvested from border areas
of flaps and recipients were cut into 5-um thick frozen sections as
well as paraffin sections at postoperative days 3, 7, 14 and 21 (n=5
pairs/time point). GFP fluorescence was inspected directly using
of a Zeiss 20T fluorescent microscope after frozen sections were
stained by 4',6-diamidino-2-phenylindole (DAPI). In order to further
investigate origination, growth directions, and anastomosis sites of
newly formed tissues including blood vessels, Hematoxylin & eosin
(HE) staining was used in frozen sections too.
The 5-um thick serial paraffin sections were also used to examine
the origination of blood vessels by immunohistochemistry analysis
[13]. Briefly, the first serial section was stained with anti-GFP
antibody (1:500, EMD Millipore, Germany) and the second one was
stained with anti-CD31 monoclonal antibody (1:100, Santa Cruz,
CA), followed by staining with a secondary antibody (Dako REALTM
EnVision TM Detection System, Peroxidase/DAB+, Rabbit/Mouse,
Denmark).
In addition, immunofluorescent staining was also exploited to
verify the origin of blood vessels. Briefly, the frozen sections were
stained with anti-CD31 antibody, followed by secondary antibody
staining and inspection under fluorescent microscope. Blood vessels
were quantified by a blinded surgeon as previously described [14].
Statistical analysis
All statistical data are expressed as mean ± standard deviation.
Data was analyzed by One-Way ANOVA and values of p< 0.05 were
considered statistically significant by means of IBM SPSS Statistics
(version 19; SPSS, Inc.).
Results
The survival of reciprocal random skin flaps
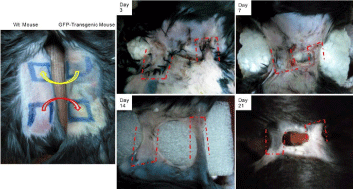
To investigate the survival of reciprocal random skin flaps, all
flaps were photographed by a digital camera at the same distance with
the same focus at postoperative days 3, 7, 14, and 21, respectively.
Almost all flaps survived well, except partial borders of two random
skin flaps suffered from ischemia as early as day 3. Red scars softened
gradually and thick hair grew slowly, but no defect or infection was
detected (Figure 1).
Revascularization by the ingrowth of capillary of random
skin flaps
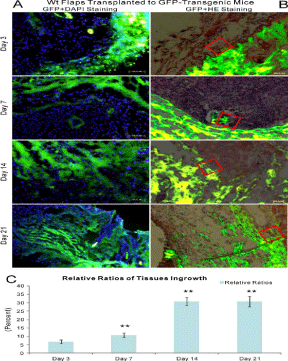
Histological examination uncovered that blood vessel ingrowth
from GFP-transgenic recipients into wt random skin flaps first
appeared at day 3 and progressively increased through day 21. The
recipient cells including blood vessels appeared not only at the
periphery, but also in the center of random skin flaps. These cells
structured as sprout-like or vessel-like cell clusters as shown by
DAPI staining (Figure 2A), which was further confirmed by HE
staining (Figure 2B). There were significant differences as to relative
ratios of GFP-positive areas to 40 um × 40 μm areas among days
3, 7 and 14, (P< 0.01), but not between days 14 and 21 (P >0.05,
Figure 2C). Furthermore, same results from serial paraffin sections
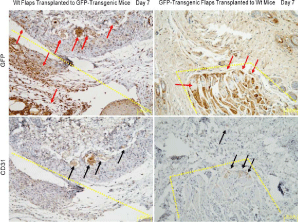
of immunohistochemistry analysis demonstrated that recipientsderived
blood vessels (GFP+/CD31+) increased in wt flaps as time
went by (Figure 3).
Figure 1
Figure 1
The design and survival of the reciprocal random skin flap model between GFP-transgenic and wt C57BL/6 mice. Arrows point to the transplantation direction of random skin flaps. Dotted frames outline the shape and position of the random skin flaps after transplantation at different time points.
Figure 2
Figure 2
The model of wt random skin flaps transplanted to GFP-transgenic recipients with a combination of green fluorescence and DAPI staining as well as green fluorescence and HE staining. A: green fluorescence and DAPI staining, B: green fluorescence and HE staining, C: relative ratios of tissue ingrowths. The red box outlined one point at the border area between random skin flaps and recipients. Newly generated capillary sprouts, blood vessels and other cells grew into random skin flaps from recipients as early as day 3 and progressively increased through day 21.
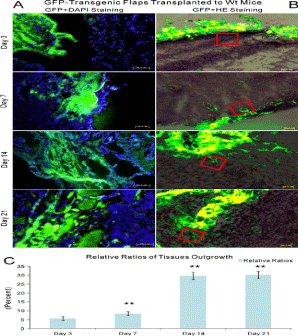
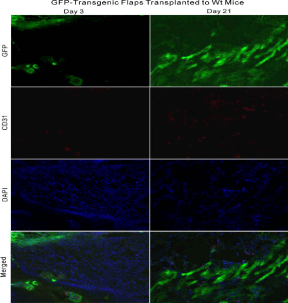
Revascularization by the outgrowth of capillary of random skin flaps
Histological examination of GFP expression revealed that newly
formed blood vessels from random skin flaps into wt recipients
emerged as early as day 3 and gradually accumulated through day
21. Donor cells including blood vessels appeared not only at the
periphery, but also in the center of recipients, taking on capillary
sprout-like or vessel-like cell clusters by DAPI staining (Figure 4A).
This phenomenon was further manifested by HE staining (Figure
4B). There were significant differences regarding the relative ratios
of GFP-positive areas to 40 um × 40 μm areas among days 3, 7, and 14 (P< 0.01), but not between days 14 and 21 (P >0.05, Figure 4C).
There were, however, no significant differences between two separate
vascular networks at each time point (P >0.05). GFP-positive tissues
(GFP+/CD31-) including blood vessels (GFP+/CD31+) progressively
increased in recipients over time on serial sections (Figure 3).
Immunofluorescent staining further approved GFP+/CD31+ blood
vessels were donor (flap)-derived vessels (Figure 5).
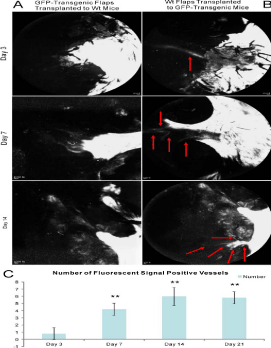
Vascular anastomoses
Vascular anastomoses of flap-derived and recipient-derived blood
vessels were first noticed at day 3 in both fluorescent angiography and
histological examination. Blood vessels full of fluorescent blood flow signals could be directly observed at day 3 in the wt C57BL/6 random
skin flaps transplanted to GFP-transgenic mice model, and they
progressively augmented through day 21 by fluorescent angiography.
There were significant differences between day 3 and other time
points (P< 0.01), but not between days 7, 14 and 21 (P >0.05, (Figure
6).
Figure 3
Figure 3
Immunohistochemistry analysis of neovascularization in serial paraffin sections. The dotted lines and frames distinguished random skin flaps from recipients. Red arrows pointed to GFP-positive tissues, and black arrows indicated CD31-positive blood vessels. GFP+/CD31+ blood vessels initially appeared at day 3 and gradually increased in wt flaps as time went by.
Figure 4
Figure 4
The combination of green fluorescence and DAPI staining as well as green fluorescence and HE staining in the model of GFP-transgenic random skin flaps transplanted to wt recipients. A: green fluorescence and DAPI staining, B: green fluorescence and HE staining, C: relative ratios of tissue in growth. Red box outlined one point at the border area between random skin flaps and recipients. Newly generated blood vessels and other cells grew from random skin flaps into recipients as early as day 3 and remarkably augmented through day 21.
Discussion
The authors demonstrated that random skin flap vascularization
is an ordered process of vascular ingrowth, blood vessels outgrowth,
followed by anastomoses in both recipients and donor flaps by using
a reciprocal random skin flap model. Through this rodent model, the authors were able to mark and trace recipient-derived tissues
including blood vessels as well as flap-derived tissues throughout the
process of revascularization in vivo, pinpointing times and locations
of all critical steps.
Through this homogenous model, the authors could easily
differentiate recipient tissues with donor structures including blood
vessels without the interference of immunologic rejection. The
authors held the idea that mutual vascular growth played a critical role in the random skin flap revascularization process. In other
words, donor endothelia outgrowth were coincident with endothelial
cell ingrowth from surrounding hosts (Figure 2 and 4). Vascular ingrowth
from GFP-transgenic recipients into wt random skin flaps emerged at
the periphery of transplanted flaps as early as day 3 and progressively
augmented through day 21 (Figure 2). Mirroring this process, in the
reciprocal random skin flap model, blood vessels grew from GFPtransgenic
random skin flaps into peripheral wt recipients as early as
day 3 (Figure 4).
Vascular anastomoses between the preexisting vasculatures
and newly formed blood vessels might be a crucial strategy in
vascularization process. The GFP-positive tissues including blood
vessels were located at the periphery and in the center of both flaps
and recipients by histological examination (Figure 2-6), indicating
that vascular anastomoses happened as early as day 3, consistent
with free skin graft revascularization by O’Ceallaigh et al. [3]. After
recipient-derived blood vessels had almost anastomosed with donorderived
vascular networks in both recipients and random skin flaps,
it was reasonable to account for GFP-positive tissues including blood
vessels increased significantly more at day 7 than day 3 (Figure 2 and 4).
These had lent support to vessel anastomoses supplying constituent
cells that could act as building blocks for new blood vessels [15].
However, in terms of GFP-positive cell clusters, there were no
obvious differences between days 14 and 21. A possible explanation was
that blood flow resumed and flap tissues became reoxygenated after
vascular anastomoses occurred between recipient vasculatures and
flap vessels. Thus, potential hypoxic signaling pathways stimulating
revascularization no longer existed and the revascularization process
ceased [8]. Furthermore, GFP-positive cell clusters had not been
found in liver or kidney (data not provided). These data suggested
that recipient-derived endothelial progenitor cells migrated into
the periphery and central area of random skin flaps [3], and vice
versa because of ischemia, but not anywhere such as liver or kidney.
In addition, a multitude of tissues besides vascular endothelial
progenitor cells showed exceedingly great growth potential under
ischemia, greatly promoting flap survival and wound coverage.
These results hinted that preexisting vascular networks might
be potential channels for the immigration of endothelial progenitor
cells and other cells into the center of random skin flaps, which
corresponded with other researchers [8]. They might also shed light
on the fact that mesenchymal stem cells or vascular endothelial
progenitor cells were these migrated cells and played an important
role in random skin flap survival as described [13 and 16].
These results could be easily extended to free flaps, bone grafts and
tissue-engineered prefabrications, which emphasize vascular/tubular
anastomoses between recipient vessels and donor vascular networks
or tissue-engineered construct tubular systems. One prerequisite
of tissue engineering was the necessity to acquire sufficient
neovascularization to support central cell survival. Therefore,
therapeutic angiogenesis, using various growth regents such as
vascular endothelial growth factor, basic fibroblast growth factors,
deferoxamine and dimethyloxalylglycine, had yielded an amazing
increase in flap survival and differentiation of endothelial progenitor
cells into vascular networks [17 and 20]. But one extremely important
prerequisite was that therapeutic angiogenesis gave priority to a
systemic way. The revascularization potential in a systemic way has
been verified by our previous job as well as other researchers’ work
[13].
Figure 5
Figure 5
The combination of green fluorescence, DAPI and CD31 staining in the model of GFP-transgenic random skin flaps transplanted to wt recipients. The CD31+/GFP+ blood vessels increasingly grew from GFP-transgenic flaps to wt recipients as time went by.
Figure 6
Figure 6
Vascular anastomoses were detected by fluorescent angiography. A: the model of GFP-transgenic random skin flaps transplanted to wt recipients, B: the model of wt random skin flaps transplanted to GFP-transgenic recipients, C: statistic result of the number of fluorescent signal-positive blood vessels. Blood vessels filling with fluorescent signal could be directly observed at day 3 in the model of wt random skin flaps transplanted to GFP-transgenic recipients. Blood vessels enhanced gradually as time went by and might reach a platform stage at day 14 and 21.
Conclusion
In conclusion, the authors initially manifested that the random skin flaps played essential roles in promoting survival and the mechanism of random skin flap revascularization occurred by means of an ordered process by the in growth and by the outgrowth of capillary buds of skin flaps, eventually vascular anastomoses between two separate vascular networks, and finally restoration of circulatory continuity through a unique reciprocal random skin flap model.
Acknowledgement
We thank Li Li, Xiao-Yu Li, Xiu-Qun Li, and Li Deng for facilitating collaborative interactions. We also extend our thanks to Dan Meng, Chang-Yong Liu, Shuang Zhang, Ji-Yuan Liu, and Meng- Jiang Yuan for their excellent technical assistance.
Role of the Funding Source
This study was sponsored by the National Natural Science Foundation of China (No.: 81400493), and Program for Innovation Team Building at Institutions of Higher Education in Chongqing in 2016.
References
- Ohmori S, Kurata K. Experimental studies on the blood supply to various types of skin grafts in rabbits using isotope P32. Plast Reconstr Surg Transplant Bull. 1960: 25: 547-555.
- Clemmesen T, Ronhovde DA. Restoration of the blood-supply to human skin autografts. Scand J Plast Reconstr Surg. 1968: 2: 44-46.
- O'Ceallaigh S, Herrick SE, Bluff JE, McGrouther DA, Ferguson MW. Quantification of total and perfused blood vessels in murine skin autografts using a fluorescent double-labeling technique. Plast Reconstr Surg. 2006: 117: 140-151.
- Lindenblatt N, Calcagni M, Contaldo C, Menger MD, Giovanoli P, Vollmar B. A new model for studying the revascularization of skin grafts in vivo: the role of angiogenesis. Plast Reconstr Surg. 2008: 122: 1669-1680.
- Henry L, Marshall DC, Friedman EA, Goldstein DP, Dammin GJ. A histological study of the human skin graft. Am J Pathol. 1961: 39: 317-332.
- Haller JA, Billingham RE. Studies of the origin of the vasculature in free skin grafts. Ann Surg. 1967: 166: 896-901.
- Zarem HA, Zweifach BW, McGehee JM. Development of microcirculation in full thickness autogenous skin grafts in mice. Am J Physiol. 1967: 212: 1081-1085.
- Capla JM, Ceradini DJ, Tepper OM, Callaghan MJ, Bhatt KA, Galiano RD, et al. Skin graft vascularization involves precisely regulated regression and replacement of endothelial cells through both angiogenesis and vasculogenesis. Plast Reconstr Surg. 2006: 117: 836-844.
- Converse JM, Smahel J, Ballantyne DL, Harper AD. Inosculation of vessels of skin graft and host bed: a fortuitous encounter. Br J Plast Surg. 1975: 28: 274-282.
- Young CM. The revascularization of pedicle skin flaps in pigs: a functional and morphologic study. Plast Reconstr Surg. 1982: 70: 455-464.
- Palmer B, Jurell G, Norberg KA. The blood flow in experimental skin flaps in rats studied by means of the 133 xenon clearance method. Scand J Plast Reconstr Surg. 1972: 6: 6-12.
- Tsur H, Daniller A, Strauch B. Neovascularization of skin flaps: route and timing. Plast Reconstr Surg. 1980: 66: 85-90.
- Zhang FG, Yao Y, Feng Y, Hua CG, Tang XF. Mesenchymal stem cells transduced by stromal cell-derived factor-1alpha augment ischemic free flaps' survival. Ann Plast Surg. 2011: 66: 92-97.
- Yao Y, Hua C, Tang X, Wang Y, Zhang F, Xiang Z, et al. Angiogenesis and osteogenesis of non-vascularised autogenous bone graft with arterial pedicle implantation. J Plast Reconstr Aesthet Surg. 2010: 63: 467-473.
- Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001: 7: 259- 264.
- Zhang FG, Tang XF. New advances in the mesenchymal stem cells therapy against skin flaps necrosis. World J Stem Cells. 2014: 6: 491-496.
- Rezende FC, Gomes HC, Lisboa B, Lucca AF, Han SW, Ferreira LM. Electroporation of vascular endothelial growth factor gene in a unipedicle transverse rectus abdominis myocutaneous flap reduces necrosis. Ann Plast Surg. 2010: 64: 242-246.
- Liu PY, Wang XT, Xin KQ, Chen CH, Rieger-Christ K, Summerhayes IC, et al. Application of AAV2-mediated bFGF gene therapy on survival of ischemic flaps: effects of timing of gene transfer. Ann Plast Surg. 2009: 62: 87-91.
- Wang C, Cai Y, Zhang Y, Xiong Z, Li G, Cui L, et al. Local injection of deferoxamine improves neovascularization in ischemic diabetic random flap by increasing HIF-1alpha and VEGF expression. PLoS One. 2014: 9: e100818.
- Takaku M, Tomita S, Kurobe H, Kihira Y, Morimoto A, Mayuko Higashida, et al. Systemic preconditioning by a prolyl hydroxylase inhibitor promotes prevention of skin flap necrosis via HIF-1-induced bone marrow-derived cells. PLoS One. 2012: 7: e42964.